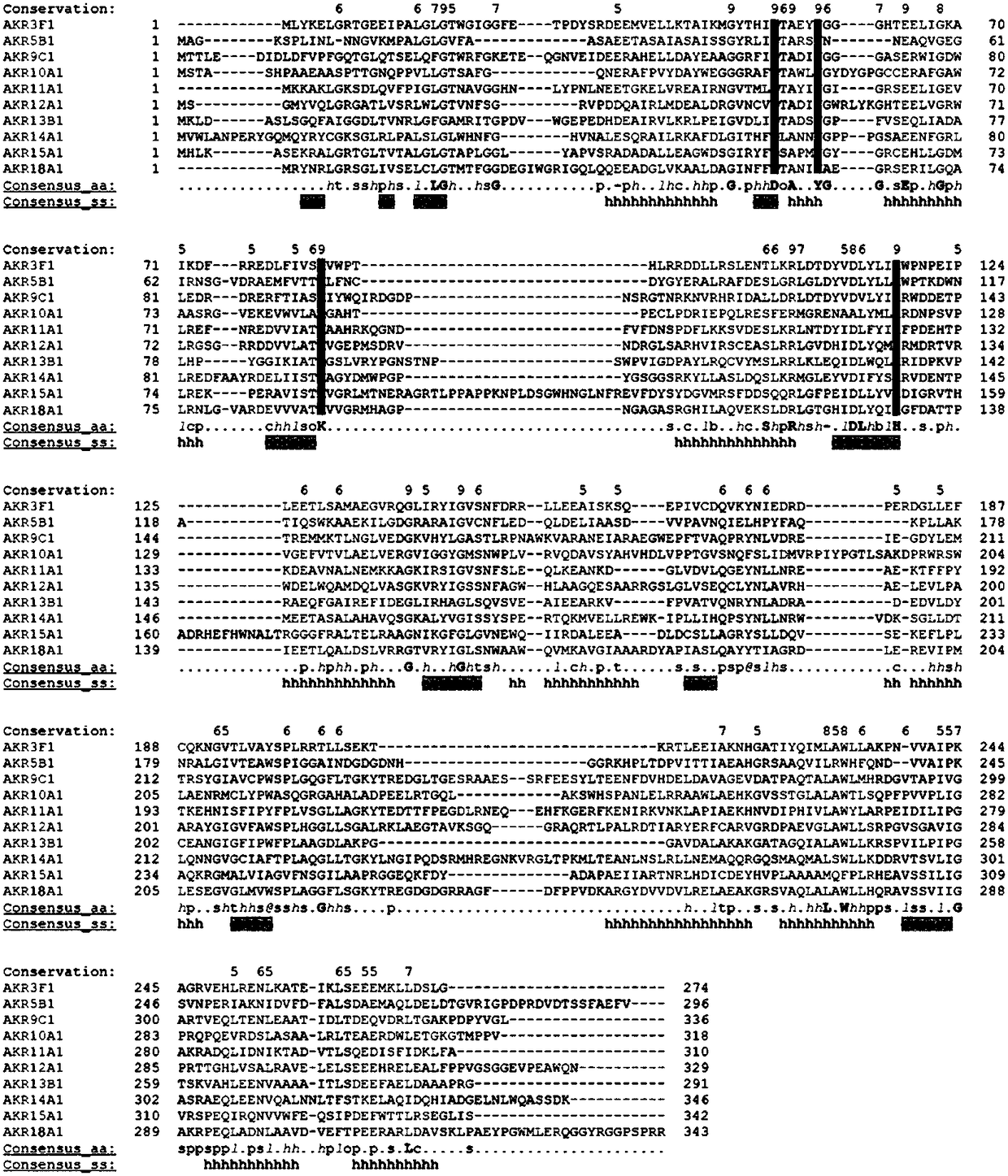
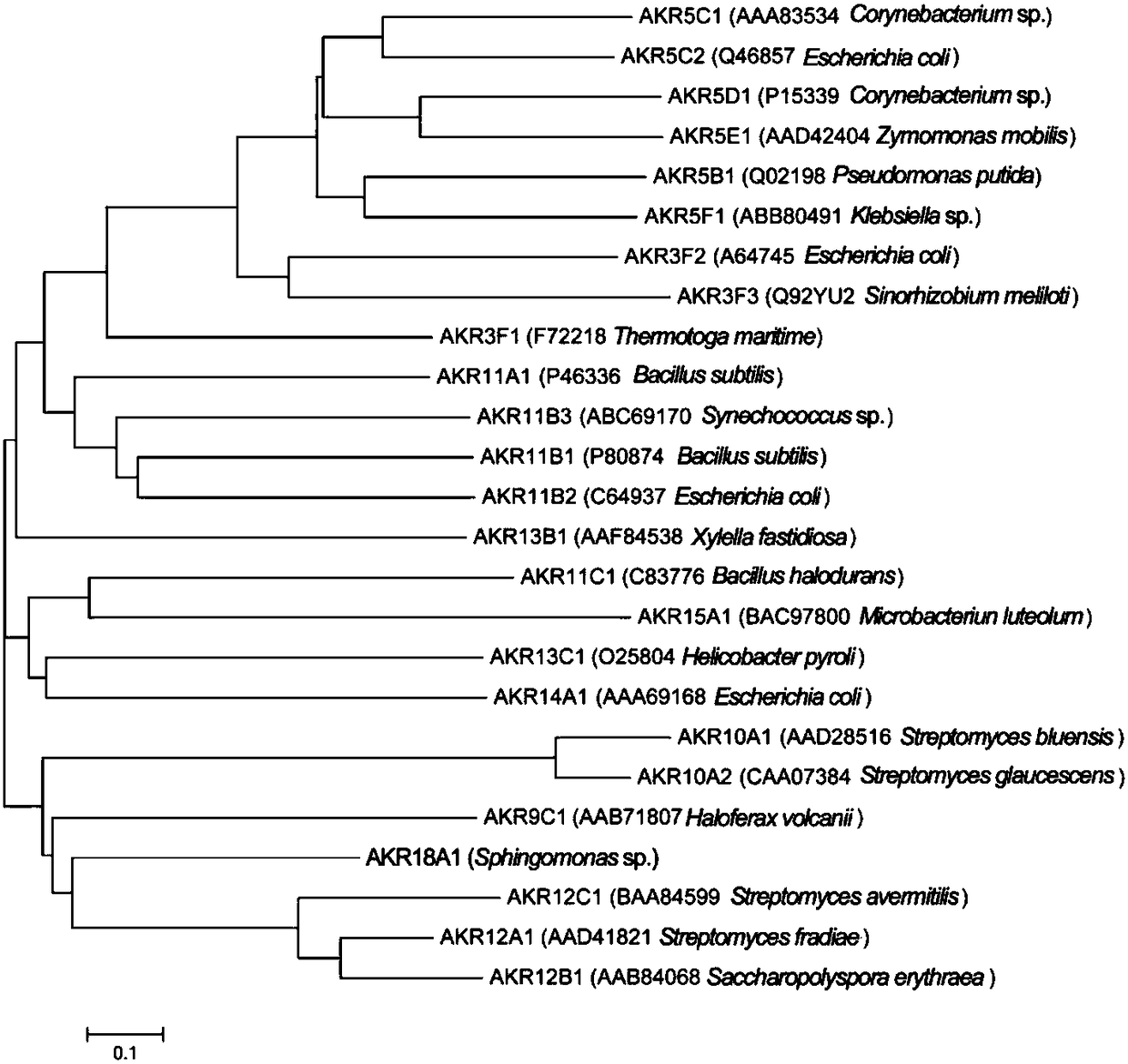
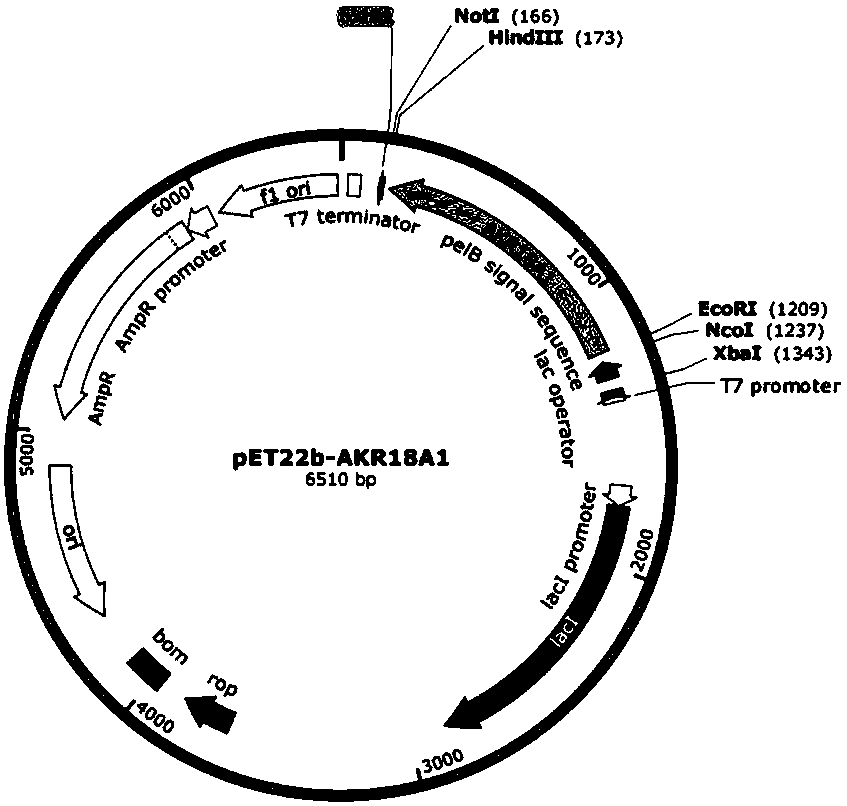
Gene AKR18A1 relevant to detoxification of fusarium toxin and toxic aldehydes and applications of gene AKR18A1
A fusarium and gene technology, applied in the direction of DNA / RNA fragments, applications, genetic engineering, etc., to achieve the effects of easy expression, simple cloning operation, and specific products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Cloning of target gene AKR18A1 and construction and transformation of prokaryotic expression vector
[0037] Target sequence cloning: Using the genomic DNA of Sphingomonas sp. degrading bacteria S3-4 (provided by the research group laboratory) as a template, the high-fidelity enzyme KOD plus (purchased from Toyobo, Japan) was amplified Target gene fragment. EcoR I and Hind III restriction sites were added to both ends of the primer, forward primer: 5′-GGAATTCGATGCGCTACAACCGGCTCGGCCG-3′, reverse primer: 5′-CCCAAGCTTGCGCCGC GGCGACGGGCCG-3′. 50μL reaction system: 5μL of 10×KOD buffer, 25mmol / L MgSO 4 2 μL, 5 μL of 2 mmol / L dNTPs, 1.5 μL of each 10 μmol / L primer, 1 μL of KOD plus (1U / μL), 1 μL of template cDNA, 5 μL of betaine, supplemented with ddH 2 O to a total volume of 50 μL. The reaction program was: 95°C pre-denaturation for 5 minutes; 95°C for 30s, 68°C for 2min, 35 cycles; 68°C extension for 10min. After sequencing the target fragment, the sequence ...
Embodiment 2
[0040] Example 2: Expression and purification of AKR18A1 protein
[0041] Induced expression of protein: culture Escherichia coli BL21 strain containing pET-22b-AKR18A1 vector in LB liquid medium, and shake it at 37°C (200r / min) to OD 600 It reaches about 0.6; add IPTG (purchased from Sigma, USA) at a final concentration of 0.4 mM, place in a shaker at 16°C (140r / min) for induction and culture for 12h.
[0042] Purification of protein: collect the cells by centrifugation, and resuspend the cells with an appropriate amount of lysis buffer (according to the ratio of 1:20-1:40). Vortex vigorously to fully dissolve the cells. Before cell disruption, 100 μM protease inhibitor PMSF was added at a ratio of 1:100 (v:v), and E. coli cells were disrupted with a high-pressure cell disruptor. The bacterial cell disruption solution was centrifuged at 16000r / min for 30min, the supernatant was collected, and all passed through the protein purification column filled with Ni-NTA matrix. Add...
Embodiment 3
[0043] Example 3: Catalysis of purified AKR18A1 protein on DON
[0044] The purified AKR18A1 protein was reacted with DON in the buffer solution, and the catalytic activity of the purified protein on DON was calculated by the change of DON content in the reaction system. In 50μL reaction system, including 6μg purified protein, 100μM DON, 2mM NADP + , add buffer to a total volume of 50 μL. After the reaction is completed, add an equal volume of methanol to end the reaction, and use high-performance liquid chromatography (HPLC) to detect the DON content in the solution. The HPLC analysis system consists of: Agilent 1200 semi-preparative high-performance liquid chromatography. Main components: Quaternary pump (1260Quat Pump VL) , autosampler (1260ALS), column oven (1260FCC), UV detector (1260VWD), automatic fraction collector (1260FC-AS), analytical column (Eclipse XDB-C18, 4.6×150mm, 5μm), half Preparative chromatographic column (Eclipse XDB-C18, 9.4×250 mm, 5 μm), operating s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com