ACE2-Albumin recombinant protein as well as preparation method and application thereof
An ace2-albumin, recombinant protein technology, applied in the biological field, can solve problems such as affecting the structure of ACE2, short half-life time of ACE2 protein, limiting the practical application of ACE2 protein, etc., to improve stability, avoid antibody-dependent enhancement phenomenon, high efficiency Clonal Screening and Effects of Transfected Cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
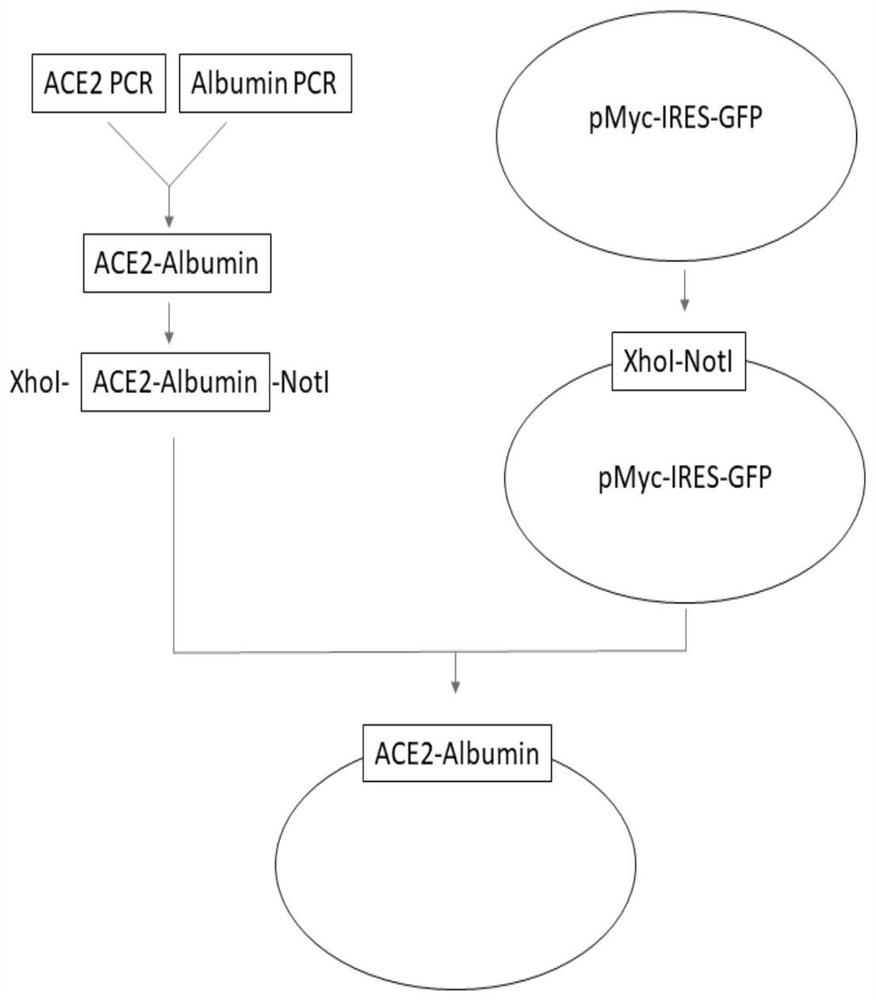
[0046] Construction of the production vector of ACE2-Albumin: the overall process is attached figure 1 shown.
[0047] The human ACE2 and Albumin gene sequences were retrieved and downloaded from the genome.ucsc.edu website, ACE2 (Genbank No: NM_001371415.1), Albumin (Genbank No: NM_000477.7) sequences.
[0048] The target gene fragment ACE2 was amplified by PCR: using intestinal cDNA as a template, using
[0049] forward primer
[0050] ACE2-5HA-F(XhoI)-ACCACC CCTCGAGATGGCCTTGACCTTTGCTTTACTGGTGGCCCTCCTGGTGCTCAGCTGCAAGTCAAGCTGCTCTGTGGGCCAGTCCACCATTGAGGAACAGGCC (SEQ ID NO. 1),
[0051] The reverse primer ACE2-5HA-R-TCTTGTGTGCAAACTCTAGGCTGTTGTCATTC (SEQ ID NO. 2) was used to amplify the ACE2 fragment by PCR. The underlined sequence is the enzyme cutting site.
[0052] The target gene fragment Albumin was amplified by PCR: using liver cDNA as a template, using the forward primer Albumin-5HA-F-CCTAGAGTTTGCACACAAGAGTGAGGTTGCTC (SEQ ID NO.4),
[0053] reverse primer
[0054]...
Embodiment 2
[0070] Production of ACE2-Albumin recombinant protein:
[0071] (1) Use FreeStyle 293-F cells with FreeStyle TM 293 medium adjusted to 1×106 cells / mL, 30 mL in total;
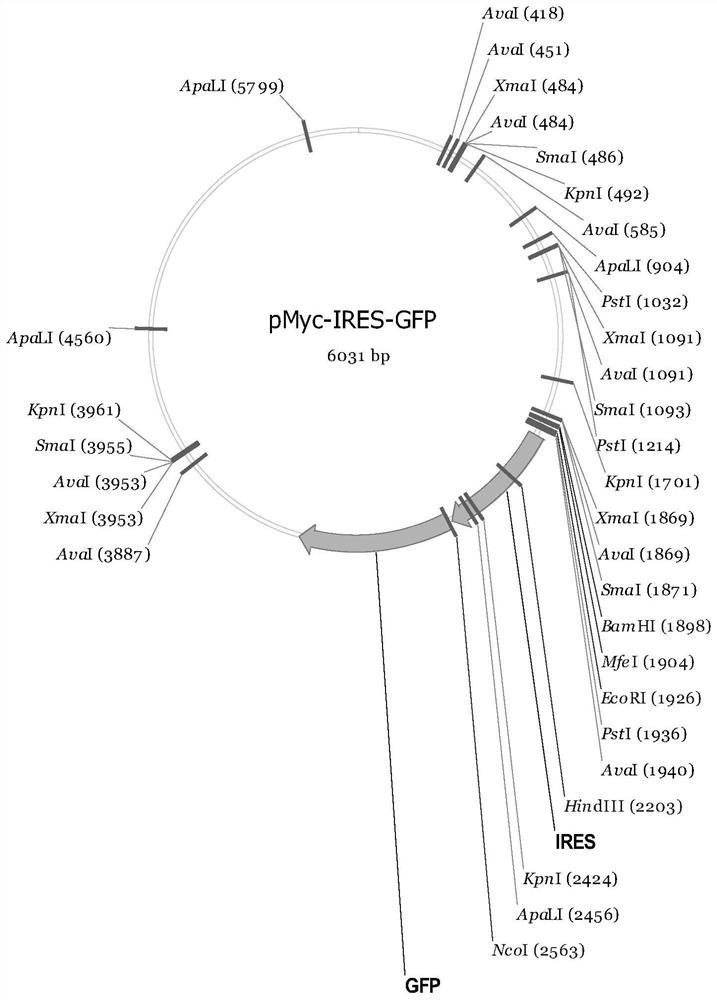
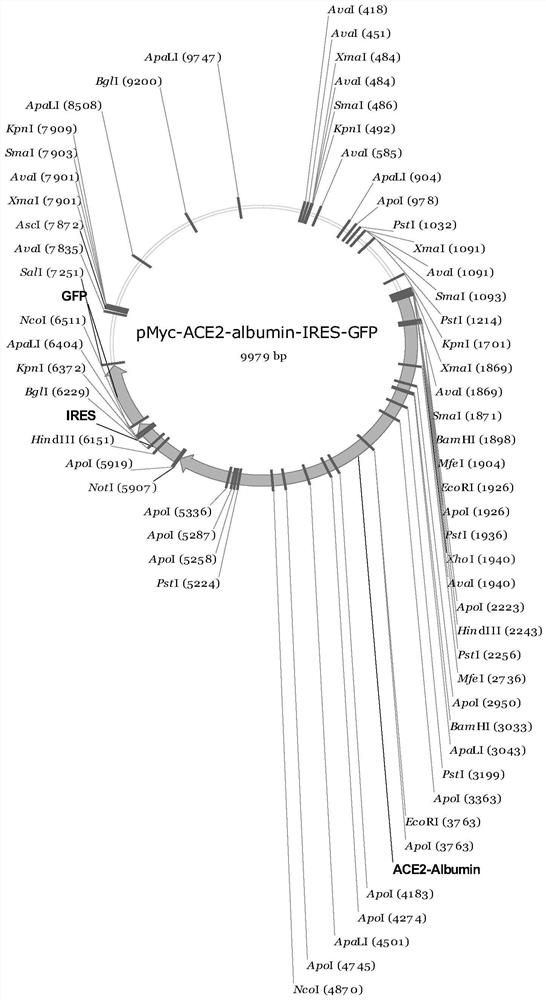
[0072] (2) Take the pMyc-ACE2-Albumin-IRES-GFP production vector 37.5ug and 0.6mL OptiPRO obtained in Example 1 TM Mix the SFM medium evenly;
[0073] (3) Take 37.5ul FreeStyle TM MAX Reagent reactant with 0.6mL OptiPRO TM Mix the SFM medium evenly and let it stand for 5 minutes;
[0074] (4) Mix the medium obtained in steps 3 and 4 evenly, and let stand for 30 minutes;
[0075] (5) Add 1.2 mL of medium obtained in step 4 into 30 mL of FreeStyle 293-F cells and culture for 7 days. On the fourth day, samples were taken to analyze the transfection of the cells. After 7 days, the ACE2-Albumin recombinant protein was collected. The sequence of the recombinant protein is shown in SEQ Shown in ID NO.11.
Embodiment 3
[0077] Purification of ACE2-Albumin recombinant protein
[0078] (1) Centrifuge 30mL of Ni-NTA Superflow Resin at 700g for 2 minutes, discard the supernatant;
[0079] (2) Add 60mL balance solution and Ni-NTA Superflow Resin, mix evenly, centrifuge at 700g for 2 minutes, and discard the supernatant;
[0080] (3) Centrifuge the cell culture obtained in Example 2 at 300 g for 5 minutes, take the supernatant and add it to the Ni-NTA Superflow Resin obtained in step 2, mix gently for 30 minutes, then centrifuge at 700 g for 2 minutes, discard the supernatant;
[0081] (4) Mix 60mL cleaning solution with Ni-NTA Superflow Resin evenly, centrifuge at 700g for 2 minutes, discard the supernatant, and wash 3 times;
[0082] (5) Mix 30mL eluent with Ni-NTA Superflow Resin evenly, mix gently for 10 minutes, then centrifuge at 700g for 2 minutes, take the supernatant, which contains CSF1-Albumin recombinant protein;
[0083] (6) Use NanoDrop to measure the concentration of ACE2-Albumin r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com