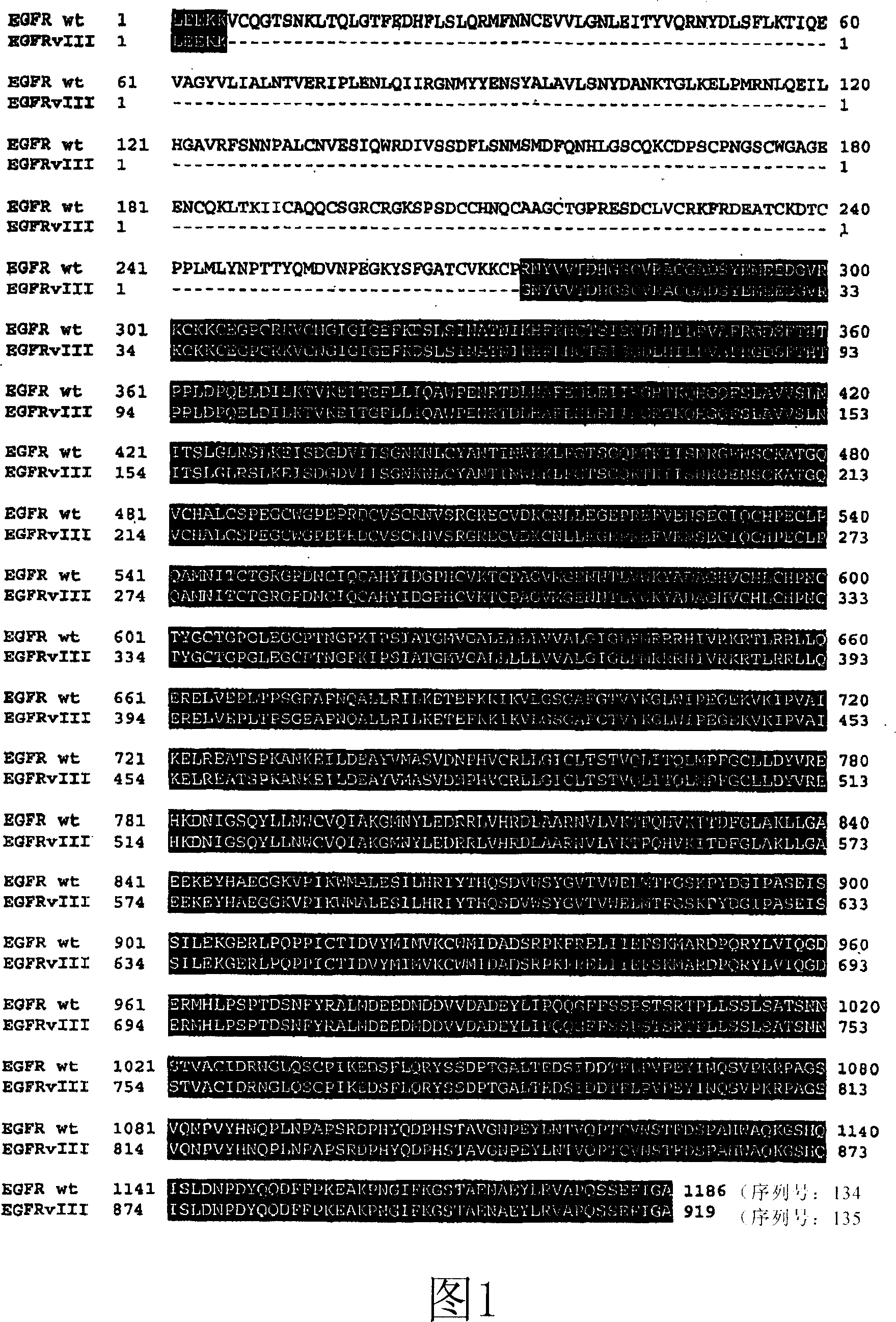
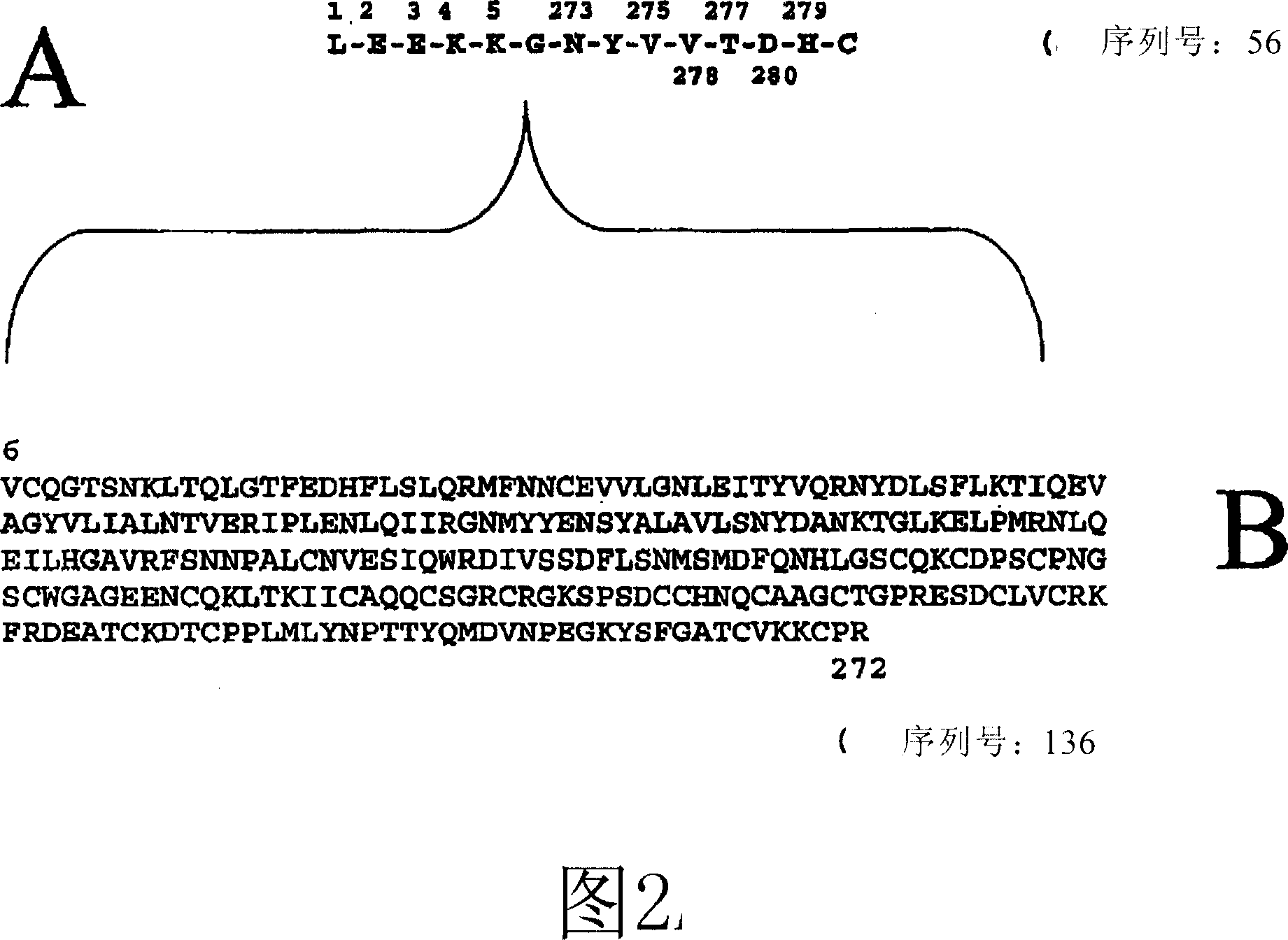
Antibodies directed to the deletion mutants of epidermal growth factor receptor and uses thereof
A technology of antibody and monoclonal antibody, applied in growth factors/growth regulators, antibodies, receptors/cell surface antigens/cell surface determinants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0214] Antibody preparation
[0215] As described herein, antibodies can be prepared using the XenoMouse(R) technology as described below. The mice are then capable of producing human immunoglobulin molecules and antibodies, but are insufficient to produce murine immunoglobulin molecules and antibodies. Techniques for accomplishing the same purpose are disclosed in the patents, applications and references disclosed herein. However, one example, inter alia, of transgenically produced mice and antibodies produced therefrom is disclosed in U.S. Patent Application Serial No. 08 / 759,620, filed December 3, 1996, and International Patent Publication June 11, 1998. The disclosures of applications WO 98 / 24893 and WO 00 / 76310 published on December 21, 2000 are incorporated herein by reference. See also Mendez et al. Nature Genetics 15:146-156 (1997).
[0216] Using this technique, fully human monoclonal antibodies can be produced against a variety of antibodies. In one example, a Xe...
example
[0223] The following examples, including experiments performed and results obtained, are provided for illustrative purposes only and are not to be construed as limiting the invention.
[0224] The initial strategy to generate EGFRvIII-specific antibodies involved immunizing XenoMouse mice with complex antigens (peptides, multiple soluble proteins, and antigen-expressing cells), followed by generation of hybrid cells by fusion or by XenoMax TM / SLAM TM The technique isolates B cells while isolating antibody producing cells. Antibody producing cells are presented to primary screening by ELISA for specificity and secondary screening by FMAT and / or FACS for cell surface binding. Internalization assays were then performed to identify antibodies that might be useful for drug delivery. The affinities of these antibodies were measured. Select specific antibodies for epitope mapping. In addition, specific antibodies are selected for in vivo and in vitro testing to analyze the effic...
example 1
[0225] Example 1' Antigen Preparation
[0226] A. Preparation of EGFRvIII PEP3-KLH antigen
[0227] Along with Example 2, the 14-mer human EGFRvIII PEP3 (LE E K K G N Y V V T D H C (SEQ ID NO: 56)) peptide was routinely synthesized by the R&D system. The PEP3 peptide was then conjugated to keyhole limpet hemocyanin (KLH) as follows: EGFRvIII PEP3 (200 meg) (R&D) was mixed with 50 meg of keyhole limpet hemocyanin (KLH; Pierce, Rockford, IL) and brought up to 165 mecl with distilled water. Add 250mcl binding buffer (0.1M MES, 0.9M NaCl, pH 4.7) and by adding 25mcl of 10mg / ml 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride Stock solutions of compounds (EDC, Pierce, Rockford, IL) crosslinked EGFRvIII PEP3 and KLH. Binding was incubated at room temperature for 2 hours, and unreacted EDC was removed by centrifugation through a 1 kDa filter (centrifugal filter, Millipore, Bedford, MA) using PBS pH 7.4.
[0228] Along with Example 3, the 14-mer human EGFRvIII PEP3 (LE E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com