Establishment method of mycoplasma ovipneumoniae indirect hemagglutination detection method
A Mycoplasma pneumoniae and method establishment technology, applied in the field of veterinary biological product detection, can solve the problems of long culture period, inapplicability of routine clinical diagnosis and epidemiological investigation, difficult detection methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Establishment of Indirect Serum Detection Method for Ovine Mycoplasma Pneumoniae Antibody
[0031] 1. Materials
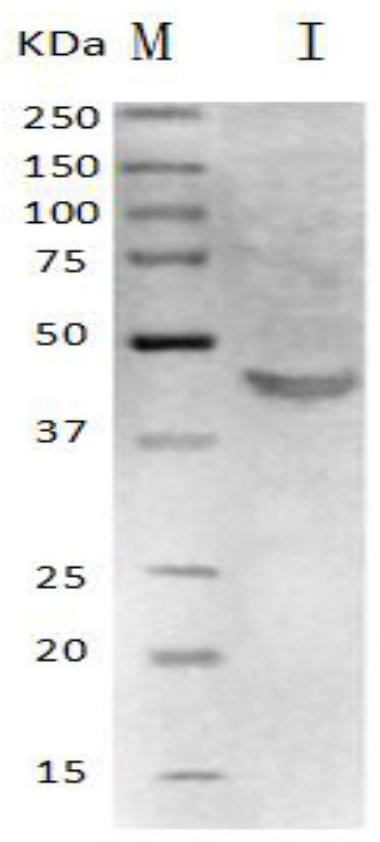
[0032] (1) Serum and strains: positive serum of Mycoplasma ovis pneumoniae and negative serum of Mycoplasma ovis pneumoniae, positive serum of Pasteurella, Staphylococcus aureus, Mannella haemolyticus and serum of Mycoplasma mycoplasma goat subspecies, positive serum of Mycoplasma hyopneumoniae, Sera of Mycoplasma filamentous subspecies were all existing materials, which were preserved at -20°C by the Laboratory of Veterinary Medicine, Southwest University for Nationalities; Escherichia coli and Salmonella factor serum were purchased from Sichuan Institute of Biological Products; C-terminal recombinant engineering bacteria (Yang Falong, Zhang Xianyu, Tang Cheng, et al. Expression and immunogenicity of C-terminal repeat region of Mycoplasma ovis pneumoniae P113 protein[J]. Chinese Veterinary Science, 2013(7):733-737.) by Stored at -20°C in the Labo...
Embodiment 2
[0060] The best reaction system and condition of embodiment 2 screening this method
[0061] ① Screen the best reaction system
[0062] Choose two rows on a 96-well V-type plate, add 10 μL of diluent to each well of the first row, and add 10 μL of Mycoplasma ovis positive serum to the first well, then perform multiple dilutions on the positive serum, and finally add the following to each well The optimal sensitization concentration is 10 μL of antigen after sensitization. Add 25 μL of diluent to each well in the second row, add 25 μL of Mycoplasma pneumoniae positive serum to the first well, then perform multiple dilutions on the positive serum, and add 25 μL of antigen sensitized at the optimal sensitizing concentration to each well after dilution. Place the 96-well V-shaped reaction plate on a shaker for 1 to 2 minutes, react at room temperature for 30 minutes to 1 hour, and record the positive serum reaction results of the two groups of experiments.
[0063] The standard ...
Embodiment 3
[0084] Embodiment 3 is to the performance evaluation of the established method specificity, sensitivity and coincidence rate
[0085] ① Specificity evaluation
[0086] With the optimal sensitizing concentration, the optimized reaction conditions were used for Escherichia coli (E.Coli) multifactor serum, Salmonella (SE) multifactor serum, Pasteurella multocida (Pm), Staphylococcus aureus (SA) It was tested with Mycoplasma mycoplasma goat subspecies serum, Mannella haemolytica serum and Mycoplasma hyopneumoniae serum. When the agglutination intensity of "++" and above appeared, it was judged as positive, and the reaction result was recorded.
[0087] Escherichia coli (E.Coli) multifactorial serum, Salmonella (SE) multifactorial serum and Pasteurella multocida (Pm), Staphylococcus aureus (SA) and Mycoplasma filamentous subspecies, Mycoplasma filamentous goat The detection results of the subspecies serum, M. hemolytica serum and Mycoplasma hyopneumoniae serum are shown in Table 4 b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com