Establishing method and application of pasteurella multocida indirect haemagglutination assay
A Pasteurella and hemagglutination test technology, which is applied in biological testing, material testing products, measuring devices, etc., can solve the problems of increasing vaccine production costs, time-consuming and labor-intensive, etc., so as to save the link of strong poisoning and attacking poisons, and the operation is simple. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Establishment of primary Pasteurella multocida indirect hemagglutination test
[0033] 1. Material
[0034] (1) Medium: Martin agar, modified Martin broth
[0035] (2) Strains: C51-2 (Pasteurella multocida, strain deposit number CVCC499), BS039 (Bronchibacterium bronchiseptica, strain deposit number HNAU0220), CMCC44149 (Escherichia coli, strain deposit number CVCC20725) , All purchased from the China Veterinary Drug Inspection Institute.
[0036] (3) Experimental animals: 1.5-2.0kg healthy and susceptible rabbits (purchased from Henan Kangda Experimental Animal Co., Ltd.).
[0037] (4) Reagent
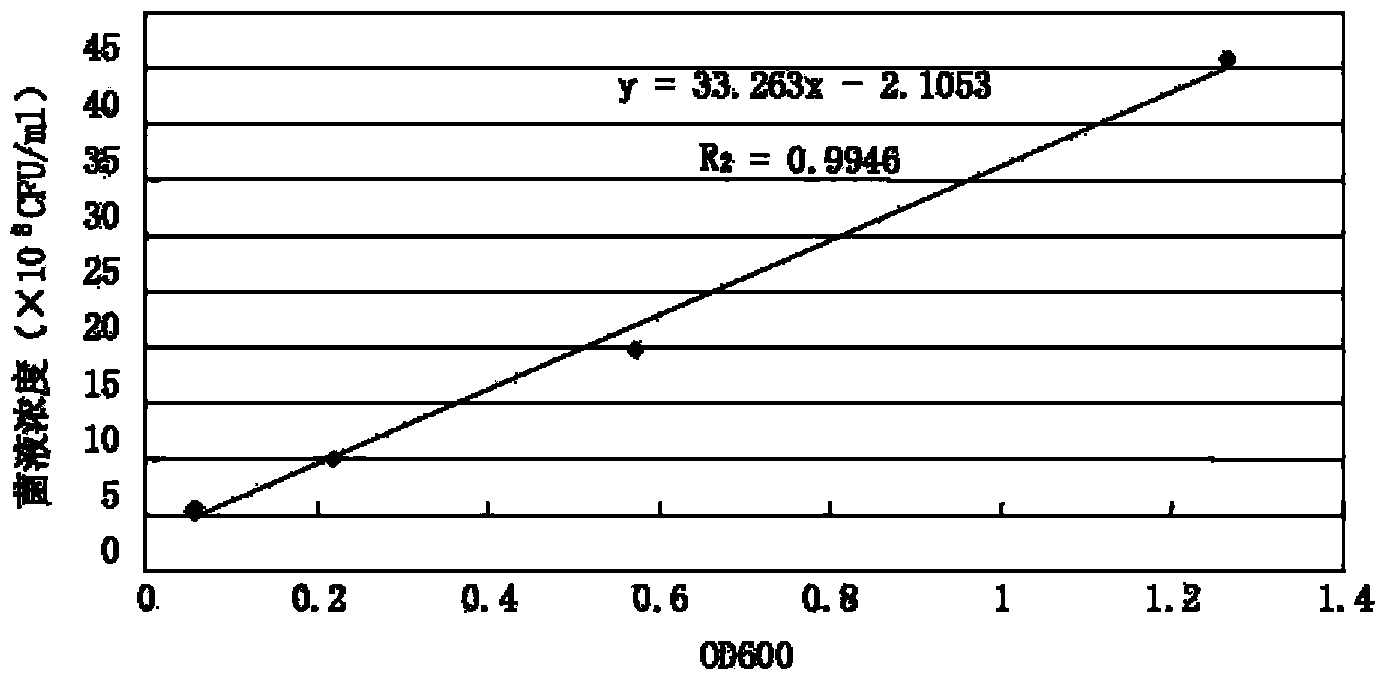
[0038] Sodium dihydrogen phosphate (Tianjin Kaitong Chemical Reagent Co., Ltd., analytical grade, 20110308); potassium dihydrogen phosphate (Chengdu Kelon Chemical Reagent Factory, analytical grade, 20100125); disodium hydrogen phosphate (Chengdu Kelon Chemical Reagent Factory, analytical grade, 20091031); glutaraldehyde (batch number 060318, Green bird imported subpackage, S...
Embodiment 2
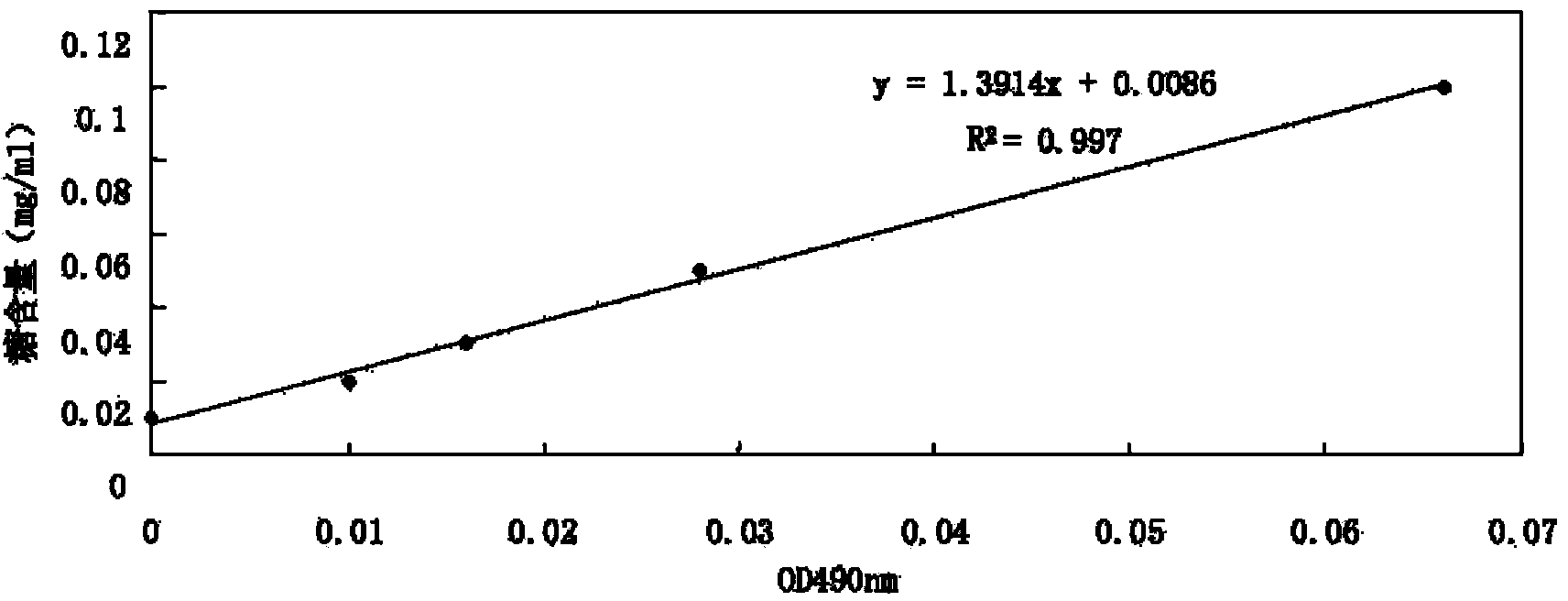
[0063] Example 2: Determination of Capsular Polysaccharide in Pasteurella multocida Capsular Antigen
[0064] 1. Material
[0065] Anhydrous glucose (sigma); phenol (imported sub-package); concentrated sulfuric acid (Luoyang Reagent Chemical Factory, 091102); 100℃ water bath, 50ml volumetric flask, 25ml volumetric flask.
[0066] 2. Method
[0067] (2) Drawing of standard curve
[0068] ① Preparation of reference solution
[0069] Take an appropriate amount of anhydrous glucose reference substance dried to constant weight at 105°C, accurately weigh it, add water to dissolve it to make a 0.5mg / ml solution, and shake it well.
[0070] ② Standard curve drawing
[0071] Precisely measure 1, 2, 3, 5, and 10ml of the above reference solution, put them in a 50ml measuring flask, and dilute each to the mark with water, and shake well. Precisely measure 2ml of the above solution and place it in a 25ml measuring flask, precisely add 1ml of 5% phenol solution, 5ml of concentrated sulfuric acid, shak...
Embodiment 3
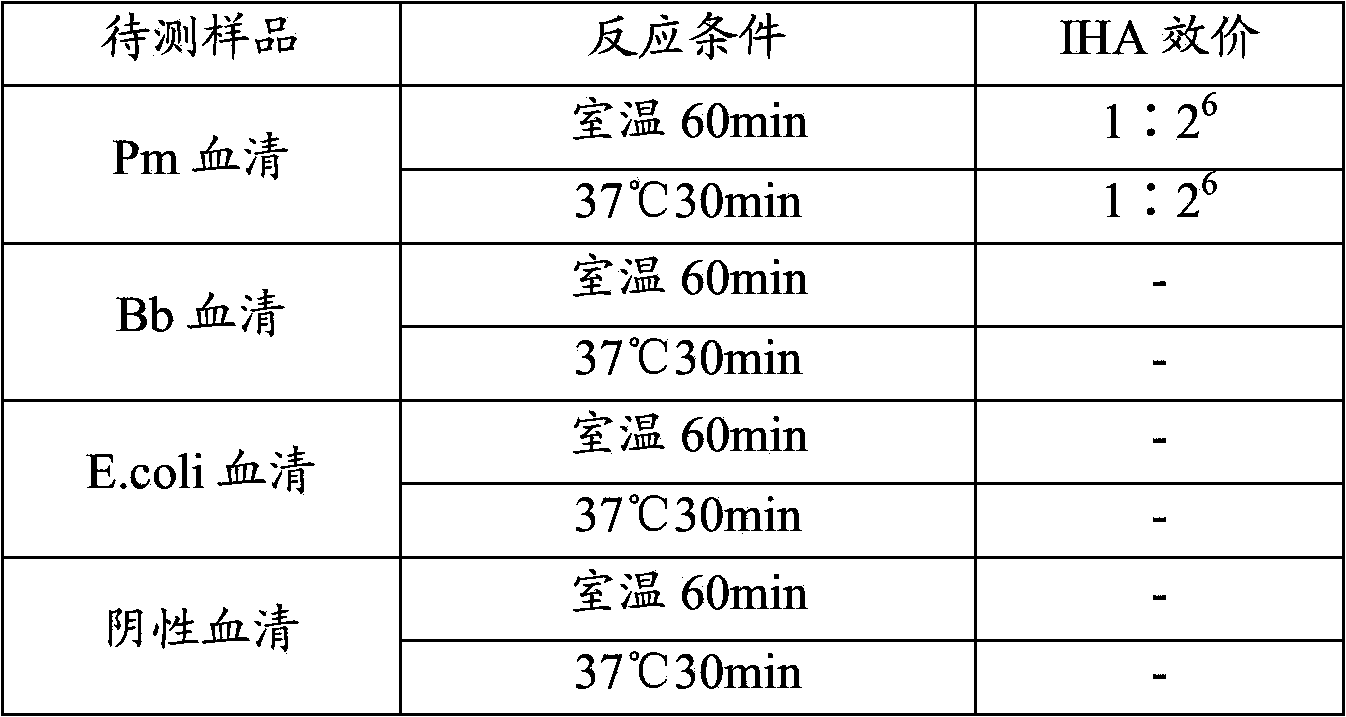
[0082] Example 3: The sensitivity and reproducibility of the Pasteurella multocida indirect hemagglutination test
[0083] The three batches of capsular antigens R1-Ag, R2-Ag, and R3-Ag were diluted 2, 4, 8, 10, 16, 20, and 25 times, respectively, and then sensitized the aldehyde-treated sheep red blood cells to perform various self-made serums. Indirect blood coagulation test. The results showed that when the polysaccharide content in the capsular antigen was 100-200 μg / ml, the indirect hemagglutination titer of Pm serum was the highest (see Table 4 for details), and the Bb serum, E.coli serum and negative serum were all negative, indicating that the The method has good specificity and reproducibility.
[0084] Table 4: Sensitivity and repeatability test results of indirect hemagglutination test method
[0085]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com