Plasma/serum circulation microRNA marker related to mlignnt melnom and application of marker
A malignant melanoma and marker technology, applied in the fields of biotechnology and medicine, to achieve the effects of improving sensitivity and specificity, accurate quantification, and easy detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The collection of embodiment 1 sample and the arrangement of sample data
[0075] The inventor has collected a large number of peripheral blood samples from patients with malignant melanoma and controls from Xijing Hospital Affiliated to the Fourth Military Medical University since 2008 (the samples used for research were collected at the same period, and the sampling, packaging, and storage conditions were uniform). For the arrangement of sample data, the inventor selected 260 samples that meet the following criteria as experimental samples for Agilent microRNA chip detection and subsequent series of qRT-PCR verification:
[0076] 1. New cases of malignant melanoma
[0077] 2. No surgery, radiotherapy and chemotherapy before blood collection, no preoperative radiotherapy and chemotherapy
[0078] 3. Healthy controls matched with the age of the cases and systematically collected demographic data, clinical data, etc. of these samples.
Embodiment 2
[0079] Agilent microRNA chip detection of miRNA in embodiment 2 serum / plasma
[0080] Among the above-mentioned eligible 30 malignant melanoma patients and 30 healthy controls, the two groups were age-matched. The two groups of people were detected by Agilent microRNA chips to obtain relevant results. The specific steps are:
[0081] 1. Use mirVana PARIS microRNA Isolation kit (mirVana PARIS miRNA Isolation kit) to extract total RNA according to the instructions provided by the manufacturer (Ambion, Austin, TX). During the extraction process, add a final concentration of 10 -4 pmol / μl artificially synthesized cel-39 (TAKARA) was used as an internal reference, and its concentration was quantitatively detected with a NanoDrop1000 spectrophotometer (NanoDrop Technologies, Waltham, MA).
[0082] 2. Label and hybridize the RNA sample. The reagents required for labeling and hybridization are included in Agilent's miRNA Complete Labeling and Hyb Kit (p / n5190-0456). The specific rea...
Embodiment 3
[0095] qRT-PCR experiment of miRNA in embodiment 3 serum / plasma
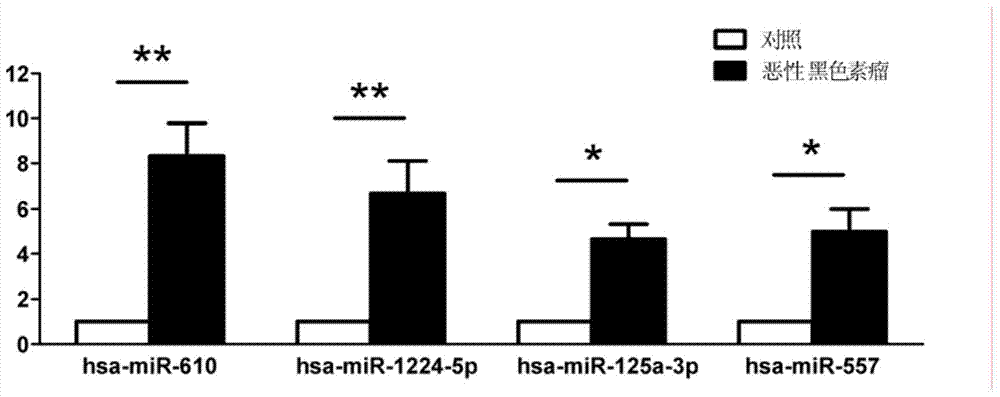
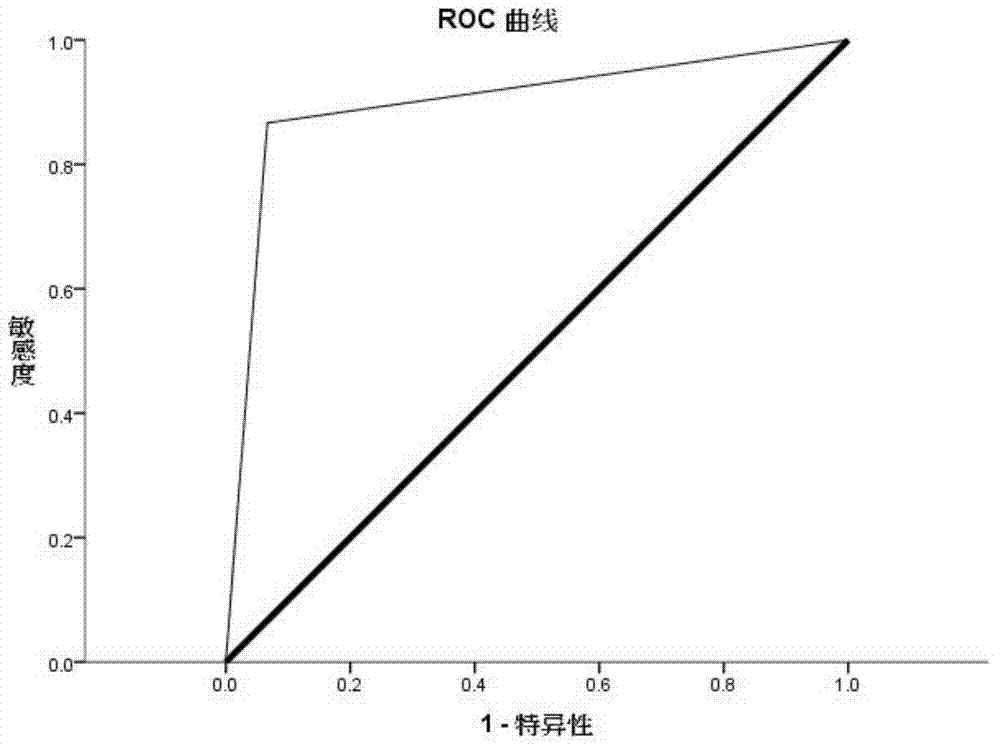
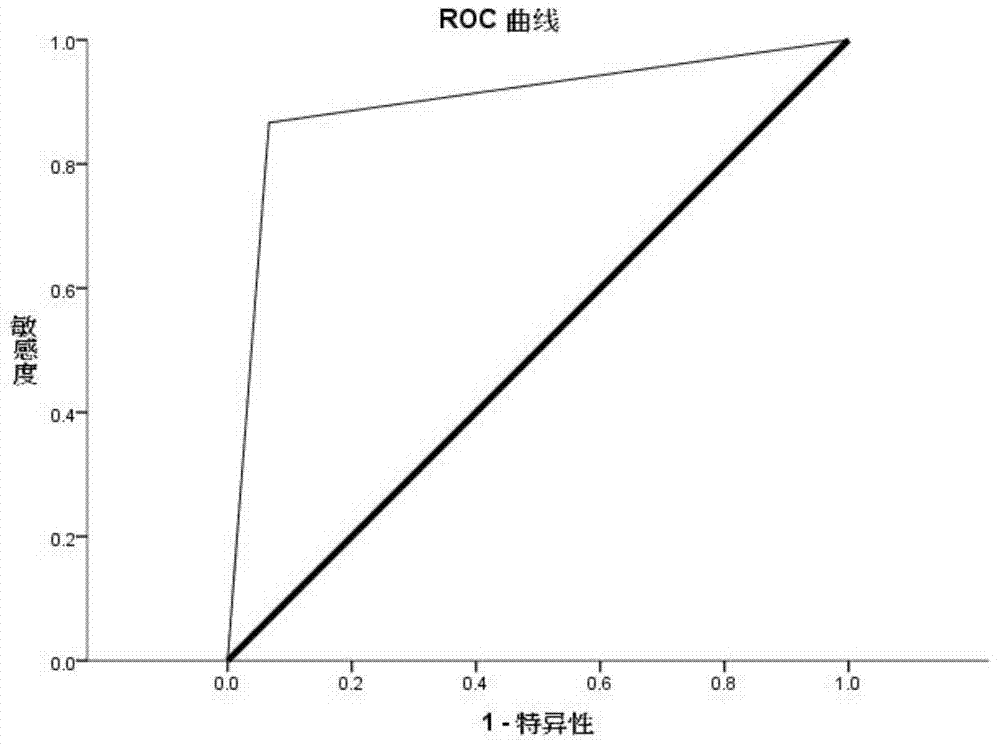
[0096] According to the above Agilent microRNA results, the top 10 miRNAs with differential expression were selected and verified by qRT-PCR in 30 cases of malignant melanoma patients and 30 cases of healthy controls:
[0097] For selected hsa-miR-610, hsa-miR-1224-5p, hsa-miR-516a-5p, hsa-miR-125a-3p, hsa-miR-202, hsa-miR-557, hsa-miR- 1182, hsa-miR-1299, hsa-miR-877, hsa-miR-371-5p and other 10 miRNAs designed qRT-PCR primers. The qRT-PCR detection of miRNA was performed on the serum individual individuals of the "malignant melanoma cases" group and the "healthy control" group. Strict quality control was implemented throughout the study. Each sample was tested three times consecutively. All assays were blinded, that is, performed without knowledge of the sample background to avoid bias. The dye method and the probe method were used for qRT-PCR detection respectively.
[0098] (1) Preparation of RNA sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com