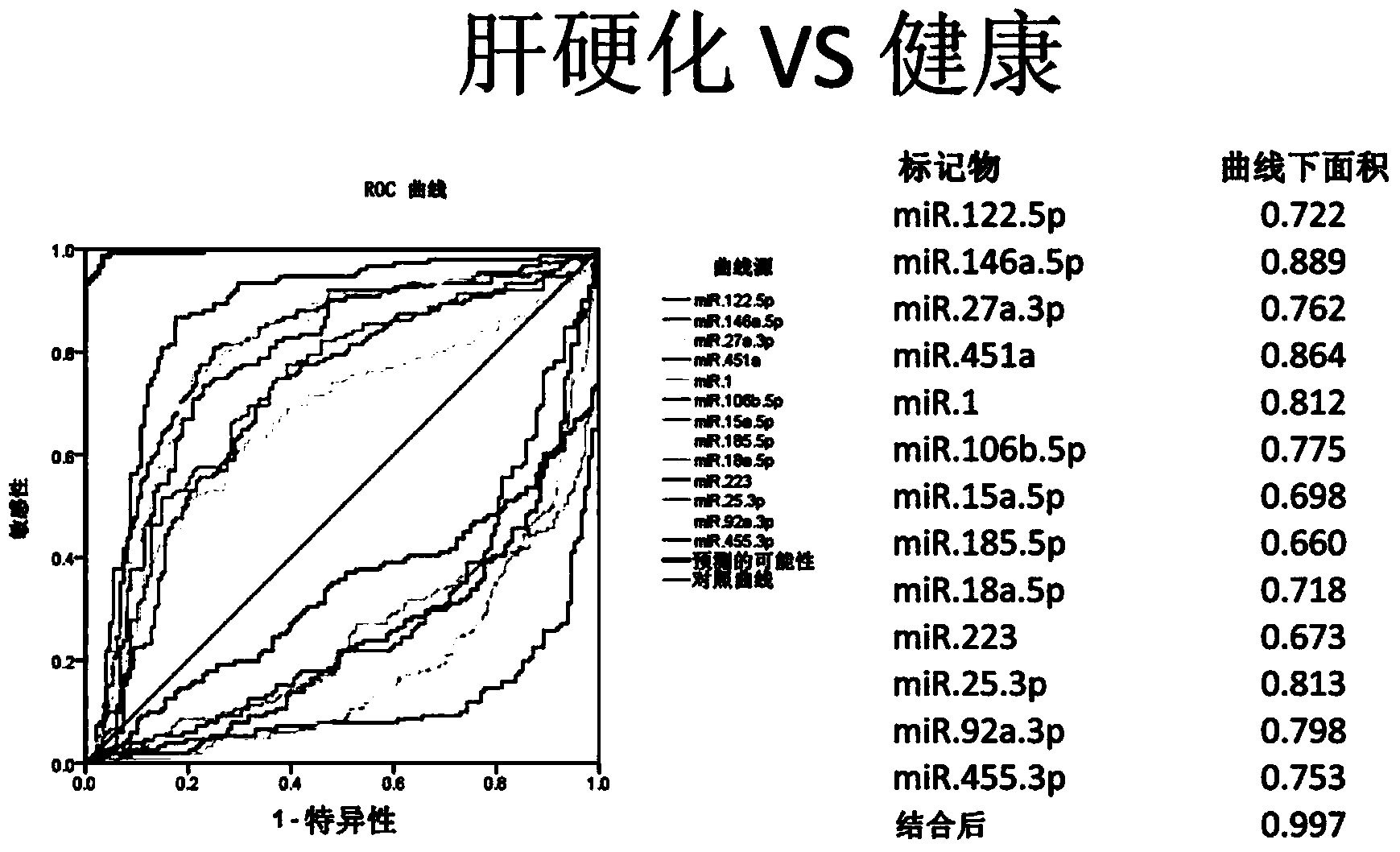
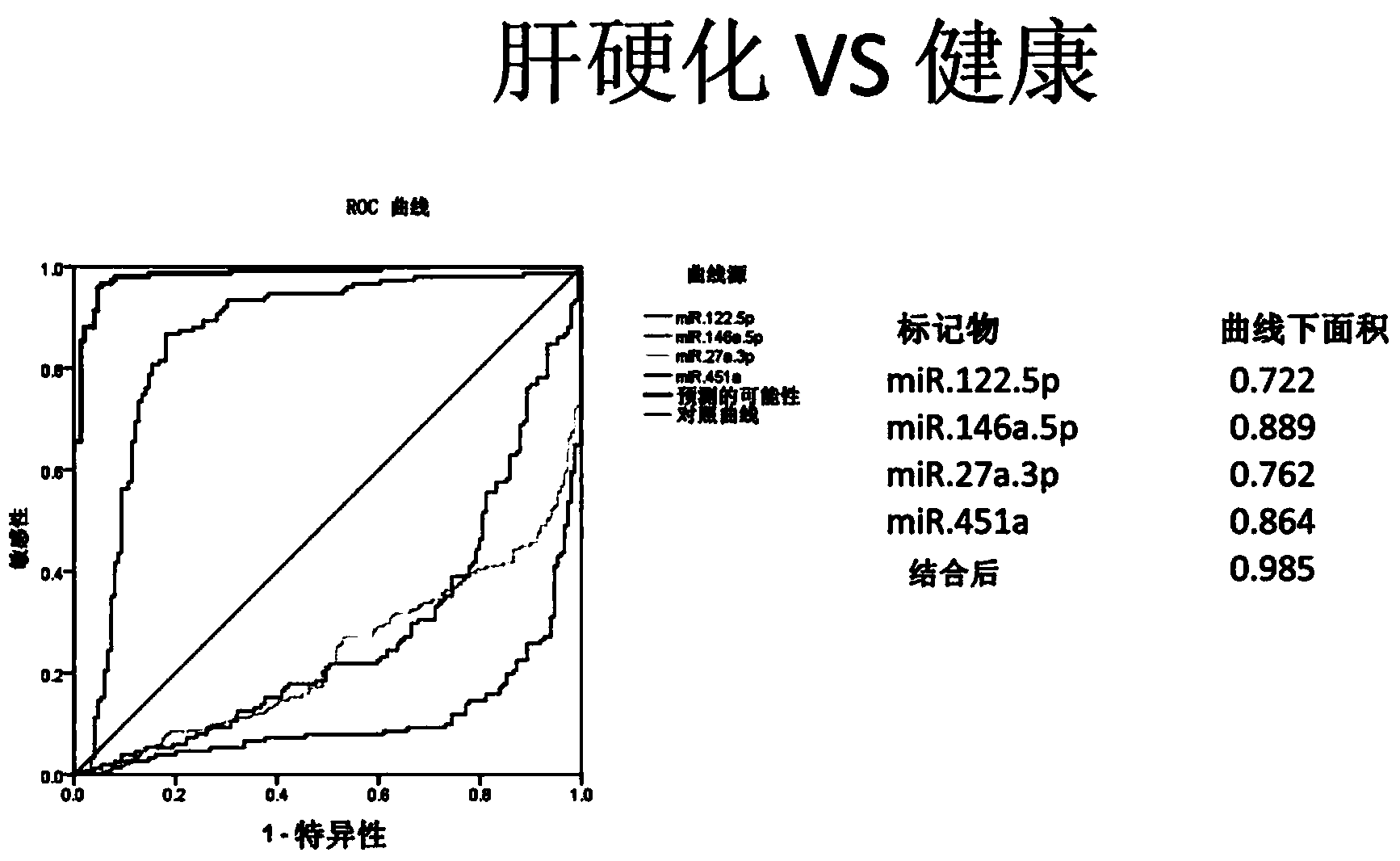
Cirrhosis microRNA molecular marker composition and application thereof
A technology of molecular markers and uses, applied in the direction of DNA/RNA fragments, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problem of no research on miRNA expression changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Collection and preparation of plasma samples
[0042] Between June 2012 and March 2014, 150 plasma samples from patients meeting the above definition of cirrhosis and 150 plasma samples from normal healthy subjects were pre-collected from Beijing You'an Hospital affiliated to Capital Medical University.
[0043] Peripheral venous blood was collected for 10 mi, anticoagulated with EDTA, and the plasma collection process was as follows: put the whole blood sample in a centrifuge at 4°C, and centrifuge at 1,500-3,000 g for 15 min. Carefully transfer the upper plasma layer to a 1.5 mL RNase-free sterile centrifuge tube with a 200 μL pipette. Label each sample. Be sure to store the plasma sample in an ultra-low temperature (-80°C) refrigerator within 4 hours.
Embodiment 2
[0044] Example 2. Extraction of total RNA in plasma
[0045] Use the RNA extraction kit (Beijing Kuangbo Biotechnology Co., Ltd.) to extract total RNA from the plasma, and add 1 μl (20 nM) of the sequence 5'-CAACCTCCTAGAAAGAGTA-3' (SEQ ID NO: 27) to each 250 μl of plasma. 1 (synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd.) to monitor the extraction quality of RNA in plasma. The concentration of extracted total RNA was determined using Thermo NanoDrop 2000c.
Embodiment 3
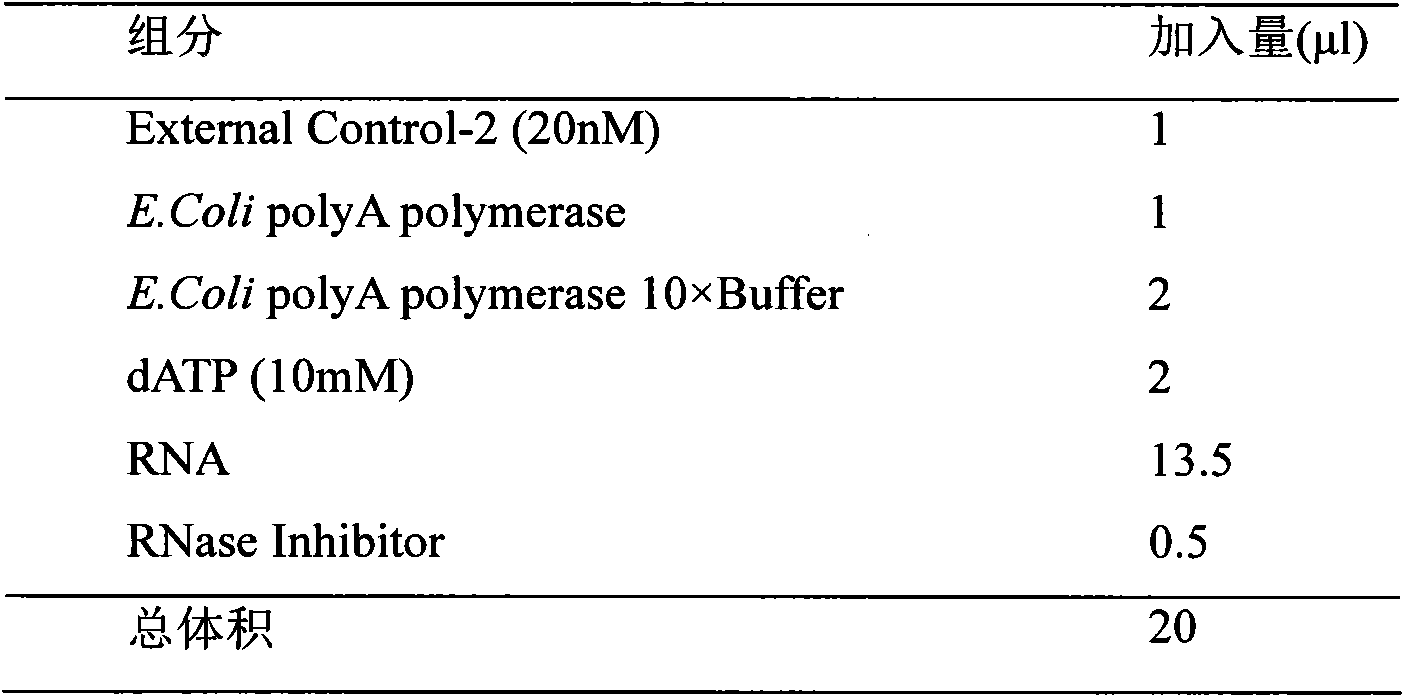
[0046] Example 3. Three-step method for quantitative detection of miRNA in plasma
[0047] (1) Add polyA tail:
[0048] i. Prepare a polyA-tailed reaction solution in an RNase-free PCR tube (Axygen, 200 μl), with a system volume of 20 μl. Add 1 μl (20nM) of External Control-2 (synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd.) with the sequence 5'-TGAGCAACGCGAACAA-3' (SEQ ID NO: 28) to each 20 μl system to monitor miRNA tailing and inversion recording quality.
[0049]
[0050] (Note: The enzymes used in this experiment are all products of Beijing Kuangbo Biotechnology Co., Ltd.)
[0051] ii. Put the PCR tube containing the prepared reaction solution into a PCR machine (Thermo) and incubate at 37° C. for 1 hour. (2) RT-PCR to obtain cDNA single strand:
[0052] i. Add 0.5 μl (0.5ng / μl) of RT-Primer (synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd.) to the reaction solution obtained in (1) with the sequence 5'-CAGTGGTATCAACGCACTCCTTTTTT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com