LncRNA gene marker and kit for ovarian cancer clinical diagnosis
A gene marker and kit technology, applied in the field of gene diagnosis, can solve the problem of lack of molecular markers and achieve the effect of improving the accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1. Detection of LincROR level in plasma. The patients with plasma samples involved in this study were all patients hospitalized in the Department of Gynecology of Nanjing First Hospital due to pelvic masses, gynecological malignant tumors, and postoperative chemotherapy for ovarian cancer. There were 220 patients in total, including 42 cases of ovarian cancer, After chemotherapy, there were 26 cases of cancer, 38 cases of endometrial cancer, 35 cases of cervical cancer, 23 cases of endometriosis, and 56 cases of benign ovarian cysts, aged 11 to 83 years. The plasma samples of the healthy control group were 30 healthy volunteers randomly selected by the Physical Examination Center of our hospital, aged 38-61 years. This study was approved by the Hospital Ethics Committee, and all subjects gave informed consent, and there was no statistically significant difference in age.
[0015] Use EDTA anticoagulant tubes to collect about 5ml of venous blood from the patient...
Embodiment 2
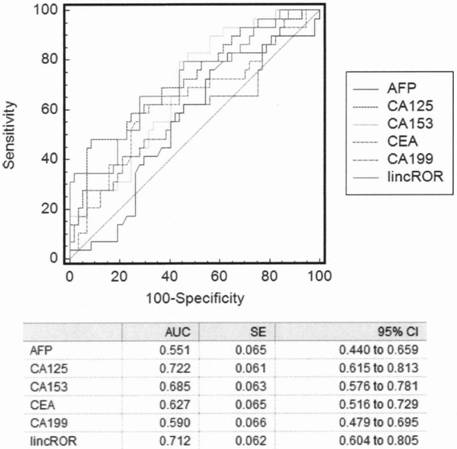
[0018] Example 2. Application of LincROR in clinical diagnosis of ovarian cancer. The present invention is used to detect the relative expression of LincROR in the plasma of healthy control women of the same age group, ovarian cysts, endometrium cancer, cervical cancer, endometriosis, ovarian cancer and ovarian cancer after chemotherapy, and the detection results show that, The expression level in the plasma of ovarian cancer patients was significantly higher than that of normal people and patients with benign ovarian cysts (3.03±4.35vs 0.31±0.24, 3.03±4.35vs 0.59±0.50, p figure 2 .
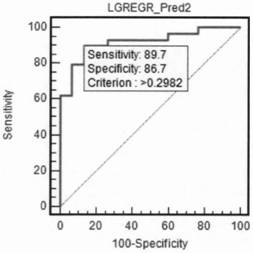
[0019] Using the relative expression of lncRNAROR in all samples of the ovarian cancer group and non-ovarian cancer (including ovarian benign cysts and healthy controls) group as the independent variable, and the group as the dependent variable, the comparison of LincROR in the ovarian cancer group and non-ovarian cancer group samples Binary logistic regression was performed on the relative inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com