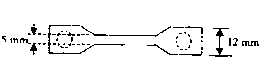
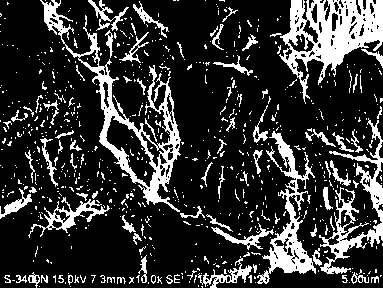
Method for preparing animal derived implantable medical biomaterial
一种动物源性、生物材料的技术,应用在医用生物材料领域,能够解决破坏三维结构、影响材料孔隙率、难以制备厚度和适应不同力学强度要求组织修复产品等问题,达到简化工艺流程、降低工艺耗时的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of decellularized porcine small intestinal submucosa matrix material
[0032] 1. Pretreatment separation and initial washing of animal tissue materials
[0033] The small intestine tissue of freshly slaughtered pigs was taken and cleaned, the submucosa of the small intestine was separated, and rinsed three times with water for injection.
[0034] 2. Virus inactivation
[0035] Use low-concentration peracetic acid-ethanol solution method to inactivate the virus. This step is carried out in a constant temperature ultrasonic cleaner that can shake the cleaning tank, wherein the volume percentage of peracetic acid is 0.05~0.2% (preferably 0.1%), The inactivation time is 1~2h (preferably 1h), the cleaning tank oscillation frequency is 30~600rpm (preferably 100~300rpm, more preferably 200rpm), the ultrasonic frequency is 20~80KHZ (preferably 20~50KHZ, more preferably 35~50KHZ, the most is 45KHZ), the temperature range is 4~40℃, and then wash 2~5 times...
Embodiment 2
[0044] Example 2: Physical and chemical properties, histology, growth factors and biological performance detection of the decellularized small intestine submucosa matrix material prepared in Example 1
[0045] 1. The physical properties of the prepared 8-layer material were tested, and the test items included suture retention, tensile strength, burst strength and porosity.
[0046] 1) Suture retention test: Method: Use 2-0 surgical sutures or stainless steel wires of the same diameter to suture 2 mm inside the edge of one end of the 8-layer material, and fix the suture or stainless steel wire with the other end of the 8-layer material. On the tension meter, stretch at a speed of 20mm / min until the suture point is torn, and record the tension when the suture point is torn. Three batches of samples were tested according to the above method. Results: The suture tensile strength was greater than or equal to 5±0.5N.
[0047] 2) Tensile strength test method: method: use a tensile ...
Embodiment 3
[0065] Example 3: Preparation of decellularized porcine dermal matrix material
[0066] The dermal tissue of a freshly slaughtered pig is taken as a raw material, and the preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com