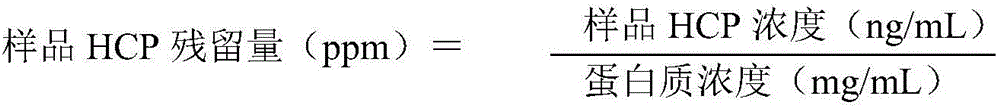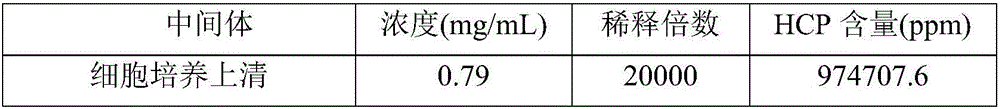Method for effectively removing host protein in monoclonal antibody downstream purification process
A monoclonal antibody and host protein technology, applied in the preparation method of peptides, chemical instruments and methods, anti-growth factor immunoglobulin, etc., can solve the problems of difficulty and reduction of HCP
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
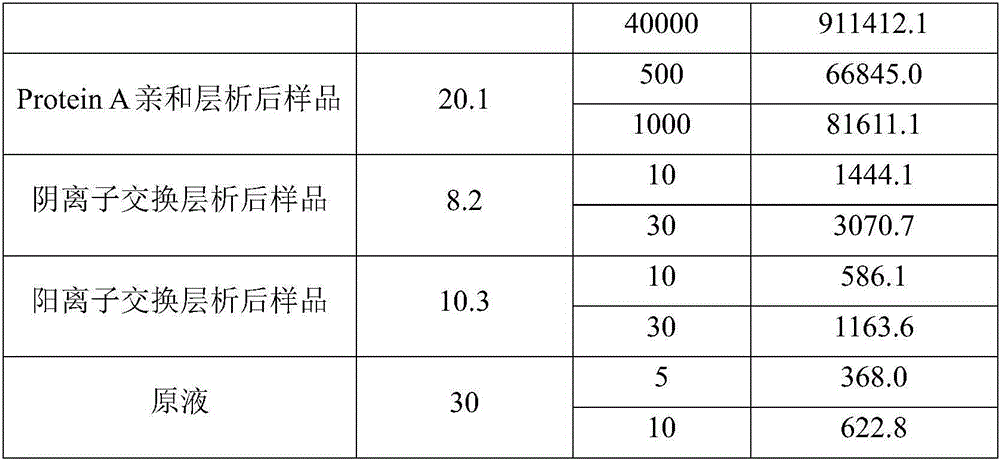
[0029] Aiming at the deficiencies in the removal of host proteins in the downstream purification process of monoclonal antibodies in the prior art, the inventors conducted in-depth research, processed the samples after virus inactivation by deep filtration, and further optimized the efficiency of Protein A affinity chromatography. conditions and depth filter conditions after virus inactivation. Using the method of the present invention, the host protein in the monoclonal antibody preparation can be effectively removed. The present invention has been accomplished on this basis.
[0030] Therefore, the present invention provides a method for effectively removing host protein in the downstream purification process of a monoclonal antibody, the method comprising (1) deeply filtering the cell culture fluid, and collecting the filtrate; (3) carry out virus inactivation to the sample obtained in step (2), and carry out depth filtration to the sample after virus inactivation, thereby...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com