Novel coronavirus vaccine based on chimeric virus-like particles
A virus and chimeric protein technology, applied in the field of human vaccines and human biological products, can solve the problem of no clear drug application for COVID-19, and achieve good immune effect, enhanced immune response, and good immune protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Another aspect of the embodiments of the present invention also provides a method for preparing the chimeric virus-like particle, which includes: transforming the recombinant vector containing the coding gene into Escherichia coli, and then culturing and post-processing to obtain The chimeric virus-like particle. The post-treatment includes operations such as cell lysis and protein purification, which are well known to those skilled in the art.
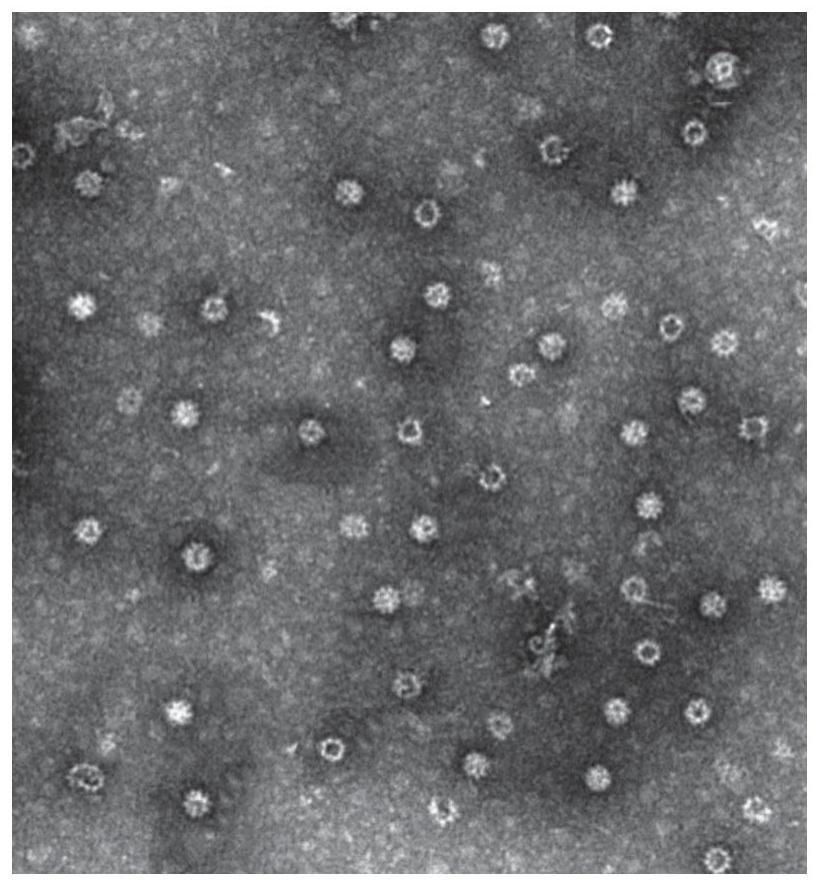
[0042] In the above embodiments of the present invention, the receptor binding region (S RBD , at least part of the sequence is shown in SEQ ID NO: 4) chimerized to the MIR region of HBcAg (at least part of the sequence is shown in SEQ ID NO: 3, wherein the 1-143rd is the assembly domain, and the 150-183rd It is the C-terminal domain CTD), which can be formed in the virus-like particles (hereinafter also referred to as S RBD / HBcAg chimeric virus-like particles) display the receptor-binding region of S protein on the surface,...
Embodiment 1
[0047] The construction of embodiment 1 recombinant escherichia coli
[0048] The codon-optimized S was synthesized at Shanghai Sunny Biotechnology Co., Ltd. RBD / HBcAg gene (SEQ ID NO: 1), inserted between NdeI / XhoI of pET-22b(+), to obtain recombinant pET-22b-S RBD / HBcAg plasmid, transform this plasmid into Escherichia coli, spread it on LB-1% agarose-amp+semi-solid medium plate and culture overnight, and the resulting clone expresses S RBD coli / HBcAg chimeric antigen.
Embodiment 2
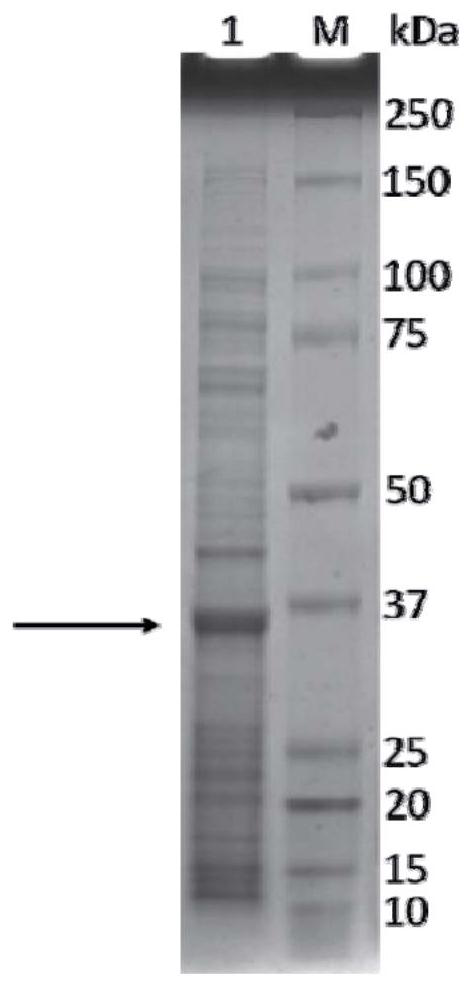
[0049] Example 2 Recombinant S RBD Preparation of HBcAg Chimeric Antigen Virus-Like Particles
[0050] 1. Pick the obtained recombinant Escherichia coli in Example 1 and cultivate it in LB medium, the culture temperature is 16°C, when the OD 600 When the value reaches 0.8, add IPTG to a final concentration of 0.1mM. After 16 hours, stop the fermentation, harvest the cells, centrifuge at 8000rpm for 15 minutes at 4°C, harvest the cells, and freeze them at -20°C until use;
[0051] 2. Resuspend Escherichia coli in 50mM Tris pH 7.5, 5mM DTT solution according to 1:4 (w:v), and add protease inhibitors, DNase 0.01mg / mL and RNase A 0.1mg / mL;
[0052] 3. Sonicate the cells in an ice bath for 15s, pause for 15s, and continue for 1min, repeating this 7-10 times;
[0053] Centrifuge at 4.4°C and 27000g for 30min, and harvest the supernatant;
[0054] 5. Slowly add ammonium sulfate to the harvested supernatant to 40% saturation, which is equivalent to a concentration of 30% (w / v), and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com