Preparation method and application of recombinant lactococcus lactis
A technology of Lactococcus lactis and lactic acid bacteria, which is applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve problems such as heavy workload, complicated separation and purification process, and complex components of inactivated vaccines, so as to improve infection rate. The effect of the ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Cloning of embodiment 1 Streptococcus agalactiae Sip gene
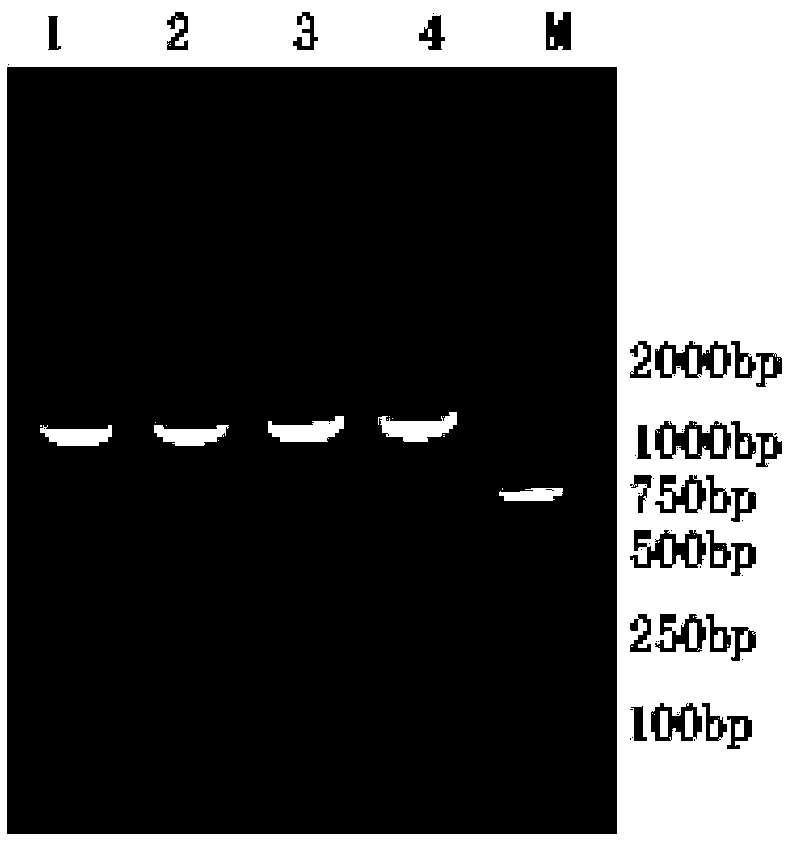
[0050] According to the Sip gene sequence of Streptococcus agalactiae published by GenBank (CP0001141), design specific upstream and downstream primers SipF (5'-CGG GGTACC CCCAAGAAACAGATACGACG-3′) and SipR (5′CCC AAGCTT TTA GTTAAAGGATACGTGAA-3′), SipF has a KpnⅠ restriction site (shown underlined), SipR has a HindⅢ restriction site (shown underlined) and 6 His tag sequences (shown in italics), primers were provided by Guangzhou Ai Synthesized by Base Biotechnology Co., Ltd. PCR amplification was performed using the tilapia-derived Streptococcus agalactiae genomic DNA as a template, and the total volume of the amplification system was 25 μL: 0.5 μL (10 mmol / L) for each of the upstream and downstream primers SipF and SipR, 0.5 μL template DNA, PremixTaq TM 12.5 μL, 11 μL of double distilled water. The PCR reaction conditions were pre-denaturation at 94°C for 5 minutes, 30 cycles at 94°C for 30s, 52°C for ...
Embodiment 2
[0051] Example 2 Preparation of Lactococcus lactis expressing Sip protein recombinantly
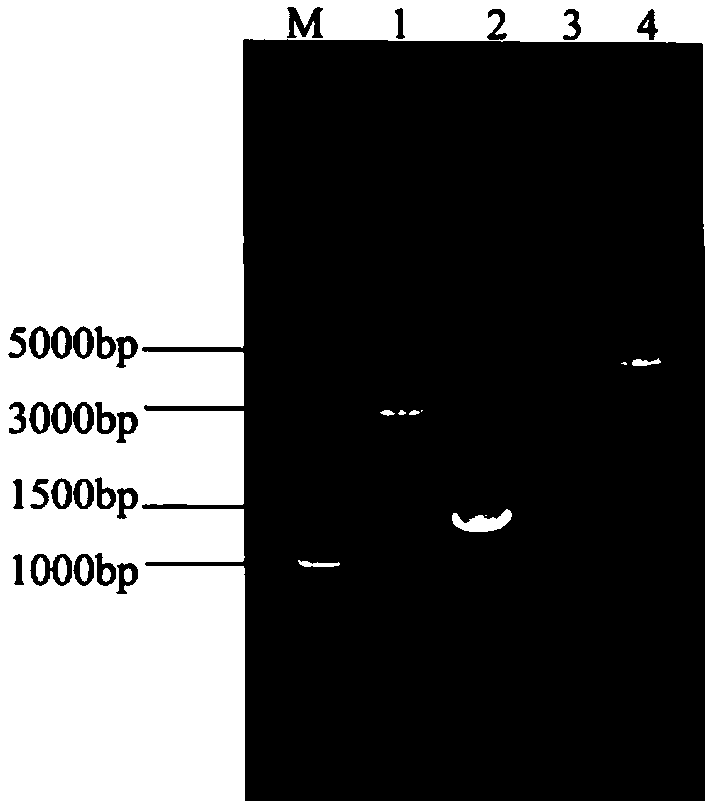
[0052] Inoculate the DH5α-pMD19T-Sip strain verified by sequencing into LB (Amp + , 100 μg / mL) liquid medium to expand culture, extract plasmid pMD19T-Sip, pMD19T-Sip plasmid, pNZ8148 plasmid and pNZ8124 plasmid were respectively digested with restriction endonucleases KpnⅠ and HindⅢ, and the digestion conditions were 37°C Incubate for 4h. The Sip gene fragment, pNZ8148 plasmid and pNZ8124 plasmid after digestion were recovered by gel, and T 4 DNA ligase was used to connect the Sip gene fragment and the pNZ8148 plasmid or pNZ8124 plasmid overnight at 16°C, and the ligated product was transformed into E.coli MC1061 competent cells to construct MC1061-pNZ8124-Sip and MC1061-pNZ8148-Sip recombinant Escherichia coli, and positive clones were detected by PCR After detection and identification by plasmid digestion, further sequencing verification was carried out. The restriction map of pNZ812...
example 3
[0054] Example 3 Sip protein induced expression and its induction condition optimization
[0055] Recombinant Lactococcus lactis NZ9000-pNZ8124-sip and NZ9000-pNZ8148-sip were inoculated into 5 mL of BHI liquid medium (Cm + , 10 μg / mL), cultured overnight at 30°C; the next day, the culture was expanded at a ratio of 1:50, and the expression was induced when the culture reached OD600≈0.4. The induction concentration optimization experiment of the inducer is to add nisin inducers with different concentration gradients (0ng / mL, 10ng / mL, 100ng / mL and 1000ng / mL), and induce at 30°C for 4h; the induction time optimization experiment is to use the best induction concentration Under the conditions, set the induction time gradient as 0h, 1h, 2h, 4h, 6h and 8h; after induction, centrifuge at 8000g for 10min to collect the bacteria, add an equal volume of PBS buffer according to the weight ratio of the bacteria at 1:1, and grind with liquid nitrogen Break up the cells, centrifuge to sav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com