Medicament for improving efficacy of oral helicobacter pylori vaccine and use thereof
A Helicobacter pylori and vaccine technology, which is applied in the direction of medical preparations containing active ingredients, antibacterial drugs, drug combinations, etc., can solve the problem of short duration, vaccines are easily destroyed by gastric acid, and vaccines cannot exert immune effects in the intestinal tract, etc. problem, to achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Detection of gastric juice pH value in Balb / c mice after intraperitoneal injection of proton pump inhibitors
[0031] 6-week-old female Balb / c mice, 18g±2g, 20 in total. Rats were grouped by random grouping method after purchase, 10 rats / cage. The feeding conditions are: feeding in a normal-grade environment, the temperature is 22±4°C, the humidity is 55±10%. For disinfection, the feed was conventional feed, and the water was obtained by autoclaving after being prepared by a pure water instrument, and they had free access to food and water.
[0032] After 7 days of transitional feeding, the mouse gastric juice pH value detection experiment was performed, and the mouse gastric juice pH value detection scheme was as follows:
[0033] Draw solution A with a sterile syringe, and inject 250 uL of solution A into each mouse by intraperitoneal injection; the composition of solution A: 0.1 mg omeprazole / mouse, and the solvent is sterile saline. 30min, 45min, 60min,...
Embodiment 2
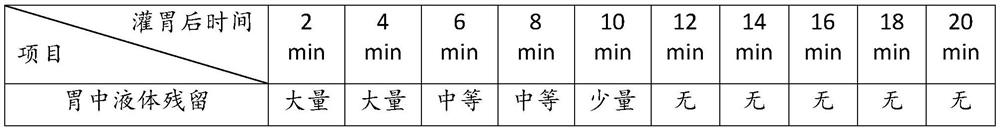
[0039] Liquid residence time in the stomach after oral gavage of Balb / c mice in embodiment 2
[0040] 6-week-old female Balb / c mice, 18g±2g, 20 in total. Rats were grouped by random grouping method after purchase, 10 rats / cage. The feeding conditions are: feeding in a normal-grade environment, the temperature is 22±4°C, the humidity is 55±10%. For disinfection, the feed was conventional feed, and the water was obtained by autoclaving after being prepared by a pure water instrument, and they had free access to food and water.
[0041] After 7 days of transitional feeding, the oral gavage experiment was carried out, and the liquid residence time in the stomach after oral gavage of Balb / c mice was tested:
[0042] Draw physiological saline with a gavage needle, and each mouse is orally gavaged with 0.3mL; 2min, 4min, 6min, 8min, 10min, 12min, 14min, 16min, 18min, 20min after the end of gavage, detect the liquid residue and excretion in the mouse stomach. Empty situation; 2 mic...
Embodiment 3
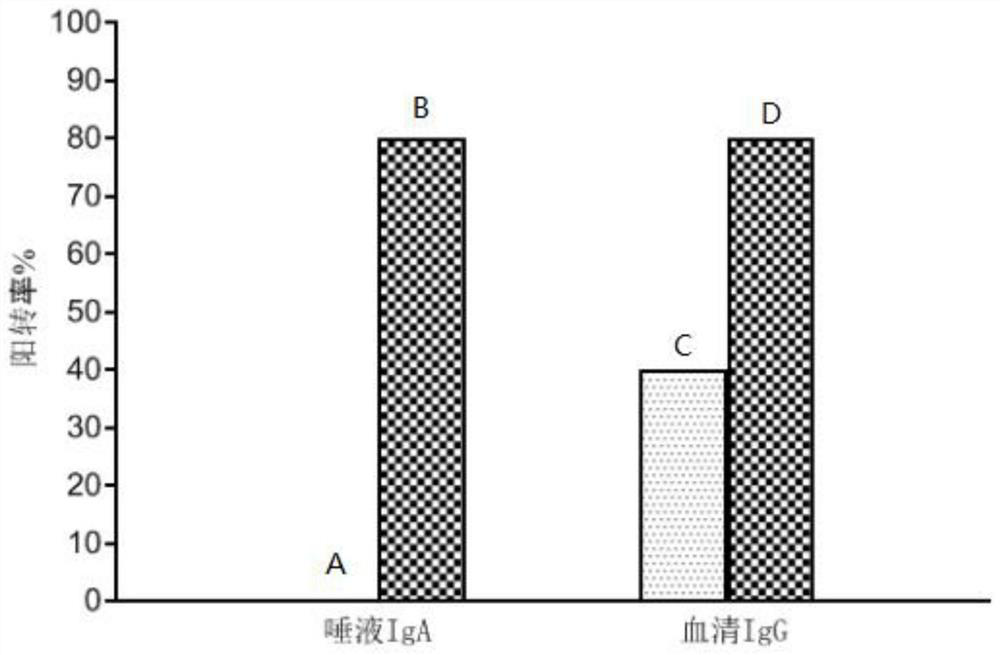
[0049] Preparations before oral Helicobacter pylori vaccine:
[0050] 6-week-old female Balb / c mice, 18g±2g, 20 in total. After the mice were purchased, the mice were grouped by random grouping method, 10 mice / cage. The feeding conditions are: feeding in a normal-grade environment, the temperature is 22±4°C, the humidity is 55±10%. For disinfection, the feed was conventional feed, and the water was obtained by autoclaving after being prepared by a pure water instrument, and they had free access to food and water. Immunization experiments were carried out 1 day after transitional feeding.
[0051] The composition of the Helicobacter pylori vaccine is as follows: A1 protein 2.5mg / mL, solvent: 1.17% sodium chloride, 0.03% sodium carbonate, 0.18% sodium bicarbonate, 5mL / 100mL glycerol, and the pH of the solution is 8.5.
[0052] Before immunization, the Helicobacter pylori vaccine was taken out from -80°C and thawed in a 4°C refrigerator for later use.
[0053] The Helicobacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com