Bivalent nucleic acid vaccine against bronchitis and preparation method and application thereof
A technology for nucleic acid vaccines and bronchitis, applied in the field of animal vaccine antigen preparation, can solve the problems of limited clinical protection effect, no effective treatment, low antigen expression, etc., to make up for the poor immunity of viruses, reduce protein purification, and improve immunity effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
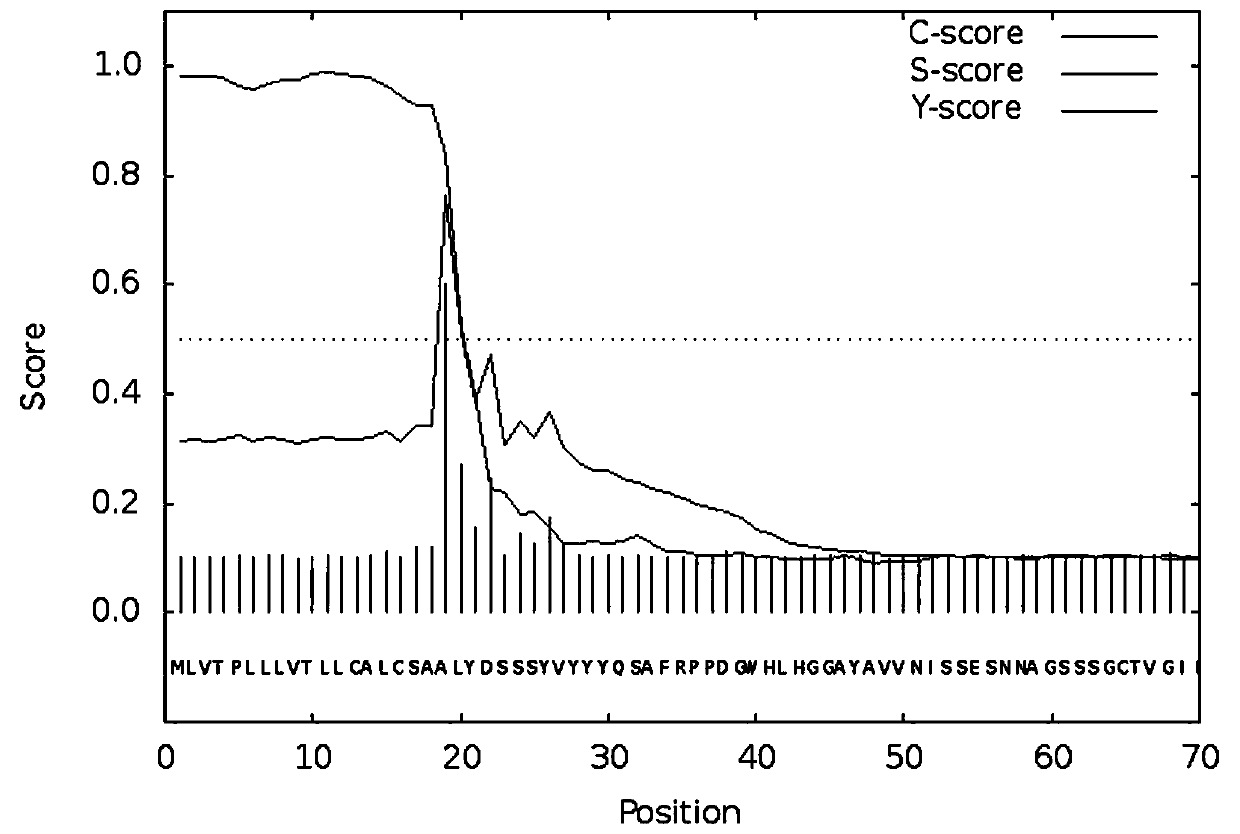
Image
Examples
Embodiment 1
[0027] The nucleic acid vaccine provided by the present invention selects Salmonella typhimurium (CMCC (B) 50115) as the nucleic acid vaccine plasmid carrier, and its advantage is that Salmonella typhimurium can colonize tissues such as chicken liver for a long time after oral immunization without being eliminated, which is It provides a long-term in vivo storage way for the vaccine, and the nucleic acid plasmid it carries can remain in the body for a long time and express antigens, providing long-term antigenic stimulation for the body, and the carrier vaccine can be obtained by fermentation and culture. Compared with conventional nucleic acid vaccines, It omits the process of plasmid extraction and endotoxin removal, and its immunization does not require special nasal drip operation, but oral immunization in the form of food additives, which is conducive to large-scale production and wide application. In this method, Salmonella typhimurium (CMCC (B) 50115) was taken, spread o...
Embodiment 2
[0036] The preparation method of bivalent infectious bronchitis nucleic acid vaccine of the present invention comprises the following steps:
[0037] According to the above sequence, the synthetic plasmid was transformed into Escherichia coli Top10 competent cells for amplification, after plasmid extraction, enrichment and amplification, electroporation into Salmonella typhimurium (CMCC (B) 50115), electroporation, and nutrient agar containing ampicillin After the plate is screened, a single colony is picked, fermented and amplified with MacConkey broth medium, and the culture collected after the culture is centrifuged is the nucleic acid vaccine.
[0038] Concrete preparation method is as follows:
[0039] 1. Plasmid acquisition and amplification
[0040] 1.1 The nucleic acid vaccine plasmid PCAGSS-M41-H120 (sequence as above) was fully synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and sequenced for confirmation. Take 2.5 μg of the plasmid for synthesis, add 100 μl...
Embodiment 3
[0062] The invention discloses the medical application of the bivalent infectious bronchitis nucleic acid vaccine for immune protection of chicken infectious bronchitis. Select 15-20 day-old chicks, and the culture is immunized by mixing materials, each 1*10 6 cfu, continuous immunization for three days, nasal drip infection of infectious bronchitis virus 15 days after immunization, the incidence rate of the immunization group was significantly lower than that of the control group.
[0063] The specific experimental method is as follows:
[0064] Take 120 15-day-old chicks (female) and raise them in isolators respectively. Each isolator has an independent filtration and ventilation system to prevent cross-infection. 30 chicks are a group, and different groups are not kept in the same isolation. Among them, the first group of 60 were fed with normal brooding feed, and the remaining 60 were fed with brooding feed with nucleic acid vaccine added for three days (1*10 7 cfu / kg), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com