Haemophilus parasuis subunit vaccine and preparation method thereof
A technology of Haemophilus suis and subunit vaccines, which is applied in the field of preventing Haemophilus parasuis disease, can solve the problems of weak antigenicity, granuloma, unsatisfactory application effect of Haemophilus parasuis vaccine, etc., and achieve saving The effect of manpower and time, and ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
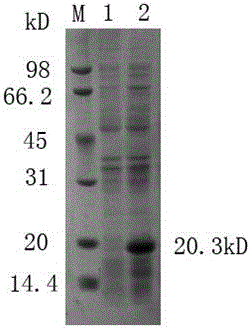
[0032] Embodiment one H. parasuis Cloning of omp16 gene and its expression in Escherichia coli BL21(DE3)
[0033] 1. Primer design and synthesis Through the bioinformatics analysis of omp16, a pair of omp16 amplification primers were designed, and the signal peptide part, that is, the N-terminal 8 amino acid sequences were removed; the length of the primer amplification fragment was 162 amino acids and a total of 486bp; -up5'-cgg gaattc gcgcctgtaggtgaaac -3' (the underlined part is Eco RI restriction site), omp16-dn 5’-ccc aagctt ttagaaacgataggac -3' (the underlined part is Hind III restriction site) primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd.
[0034] , H. parasuis The recovery and extraction of the genome take the freeze-dried H. parasuis Bacteria, streak inoculated on TSA solid plate, set at 37°C, 5% CO 2Incubated overnight. On the second day, single clones of activated bacteria were picked and inoculated in TSB medium, and cultured...
Embodiment 2
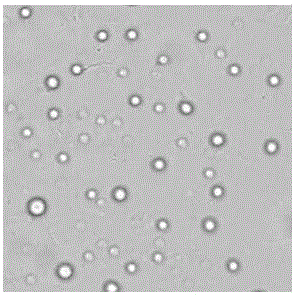
[0049] Example 2 Preparation of Haemophilus parasuis Omp16 subunit microsphere vaccine
[0050] 1. Preparation of microsphere vaccine by emulsification-ion cross-linking method. Dissolve the purified and refolded Omp16 recombinant protein in 8.8 ml of PBS at pH 8.0 to prepare a solution with a concentration of 200 mg / ml, mix it with 10 ml of 2% sodium alginate solution, add 1.2 ml of Tween 80, and stir well mix. Add 28.8 ml of olive oil and 1.2 ml of Span-80 to the mixture, stir at 1500 r / min for 20 min, and fully emulsify; add the emulsified solution dropwise to 100 ml of 8% CaCl 2 In the solution, stir at 1200 r / min for 30 minutes to cross-link and solidify; transfer the solidified microsphere suspension to a centrifuge tube, centrifuge at 1500g for 10 min, discard the upper liquid, and use 10ml 0.1 mol / L sodium acetate buffer for microsphere precipitation (pH4.5) washed 3 times, suspended with the same buffer; take 5ml of microsphere suspension into a beaker, add to 20ml,...
Embodiment 3
[0053] Example 3 Efficacy Test of Haemophilus parasuis Subunit Vaccine
[0054] 1. Mouse immune test. Fifty 8- to 10-week-old clean Kunming mice (purchased from the Animal Experiment Center of Fujian Medical University) were divided into 5 groups, 10 in each group. The immunization procedures of each group are shown in Table 2. The injection group was immunized twice with an interval of 14 days; the oral gavage group was immunized three times with an interval of 14 days.
[0055]
[0056] Fourteen days after the last immunization, the orbital venous blood of the mice was collected, the serum was separated, and stored at -20°C for future use.
[0057] Serum-specific antibody detection. Use the purified and refolded Omp16 protein of the present invention to coat the ELISA plate to establish detection H. parasuis Antibody indirect ELISA method. Omp16 protein was diluted with coating solution (0.05 mol / L carbonate buffer, pH 9.6), added to the ELISA plate (200 ng / 50 μl / we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com