Application of mycoplasma hyopneumoniae antigen in prevention and treatment of porcine respiratory disease complex
A technology of Mycoplasma hyopneumoniae and Mycoplasma pneumoniae is applied in directions such as bacterial antigen components, antibacterial drugs, etc., and can solve problems such as reports of no preventive and therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Mycoplasma hyopneumoniae Antigen
[0030] 1. Preparation of Mycoplasma hyopneumoniae seeds
[0031]After unsealing the freeze-dried strains, inoculate them in liquid medium at 10% (V / V) inoculum amount, culture them with shaking at 37°C for 3 to 7 days, and harvest them when the pH value drops from 7.5 to 6.8. seed. Take the first-grade seeds and inoculate them in liquid medium with 5% (V / V) inoculum amount, culture them with shaking at 37°C for 3-7 days, harvest when the pH value drops from 7.5 to 6.8, and use them as second-grade seeds after pure inspection.
[0032] The formula of the liquid medium: 300ml of beef heart extract (BD company), 360ml of double distilled water, correct the pH value to 7.4, and sterilize at 121°C for 15 minutes. Then add the following filter-sterilized ingredients: Hank's balanced salt solution (10×) 40m1, 0.25% (W / V) phenol red 10m1, horse serum 200m1, 5% (W / V) hydrolyzed milk protein 100m1, 25% (W / V) Yeast ext...
Embodiment 2
[0042] The preparation of embodiment 2 mycoplasma hyopneumoniae vaccine
[0043] The specific formulation of the vaccine is shown in Table 1.
[0044] Table 1 Specific formulation of the vaccine
[0045]
[0046] The specific configuration process is as follows:
[0047] According to the formula in Table 1, 10ml of the inactivated Mycoplasma hyopneumoniae HN0613 strain antigen solution prepared in Example 1, 80ml of sterile PBS buffer solution with a pH of 7.2, and 10ml of Gel 01 adjuvant (Sebic, France) were added to a sterile beaker in sequence. SEPPIC company), stirred at a speed of 500 rpm for 10 minutes at 37°C to obtain 100ml of vaccine.
[0048] Take 2ml as 1 portion / bottle, divide into packages, cover the bottle cap, and press the aluminum cap. The content of Mycoplasma hyopneumoniae was 2×10 8 CCU / Touquan.
[0049] In this embodiment, Mycoplasma hyopneumoniae is not limited to the HN0613 strain, and other pathogenic Mycoplasma hyopneumoniae strains (such as ot...
Embodiment 3
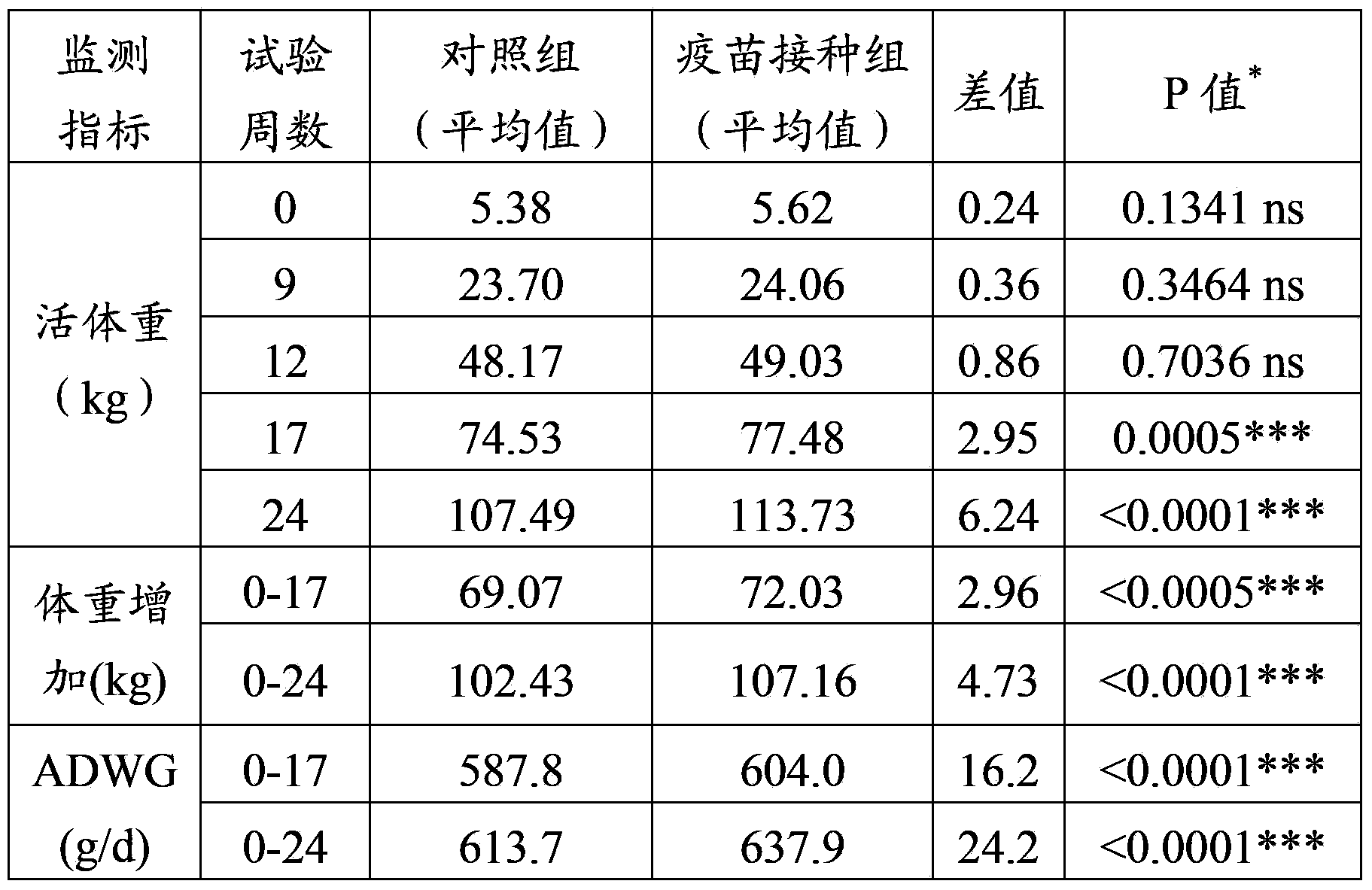
[0050] Embodiment 3 Therapeutic efficacy test of the vaccine of the present invention in PRDC pigs
[0051] In this experiment, 536 commercial pigs were included, and these pigs were divided into 2 groups randomly according to body weight and whether they were born in the same litter, namely, the vaccination group (276 pigs) A and the control group (260 pigs) B.
[0052] Group A: On the first day of the test (the 0th day), about 14 days old pigs were inoculated intramuscularly with the vaccine prepared in Example 2 once, the dose was 2ml / head, and the same vaccine and dose were used for the second time after an interval of 2 weeks. waived.
[0053] Group B: The control group was untreated pigs.
[0054] The experiment was terminated at the end of fattening (test week 24).
[0055] The parameters recorded
[0056] The parameters are documented as follows:
[0057] (1) Individual body weight (all animals)
[0058] (2) Dead pig rate (all animals)
[0059] (3) Clinical manif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com