A kind of vaccine composition and its preparation method and application
A vaccine composition and composition technology, applied in the field of vaccine composition, can solve the problems that antibacterial drugs are difficult to work, vaccines are difficult to achieve immune effect, and pig immunity is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 porcine reproductive and respiratory syndrome virus live vaccine
[0056] In this example, the spinner bottle culture method was used. Marc-145 cells were expanded and cultured in spinner bottles with cell growth medium (MEM medium containing 5% bovine serum, pH 7.25). The cells were cultured until the fifth day, and the PRRS virus was inoculated according to the M.O.I. of 0.001. After culturing until the third day after inoculation, the virus liquid was harvested and stored at -20°C. According to the highly pathogenic porcine reproductive and respiratory syndrome live vaccine (JXA1-R strain) testing procedures. Determination of virus content was 10 8.0 TCID 50 / ml.
[0057] Mix the qualified virus liquid in the same container, add 2% gelatin, 5% dextrin, 10% trehalose, 2% polyvinylpyrrolidone (PVP), 2% bovine serum at a ratio of 1:1 (V / V) Albumin, 0.164% Potassium Dihydrogen Phosphate, 0.052% Disodium Hydrogen Phosphate and water a...
Embodiment 2
[0059] Embodiment 2 Haemophilus parasuis disease, Mycoplasma hyopneumonia inactivated vaccine preparation
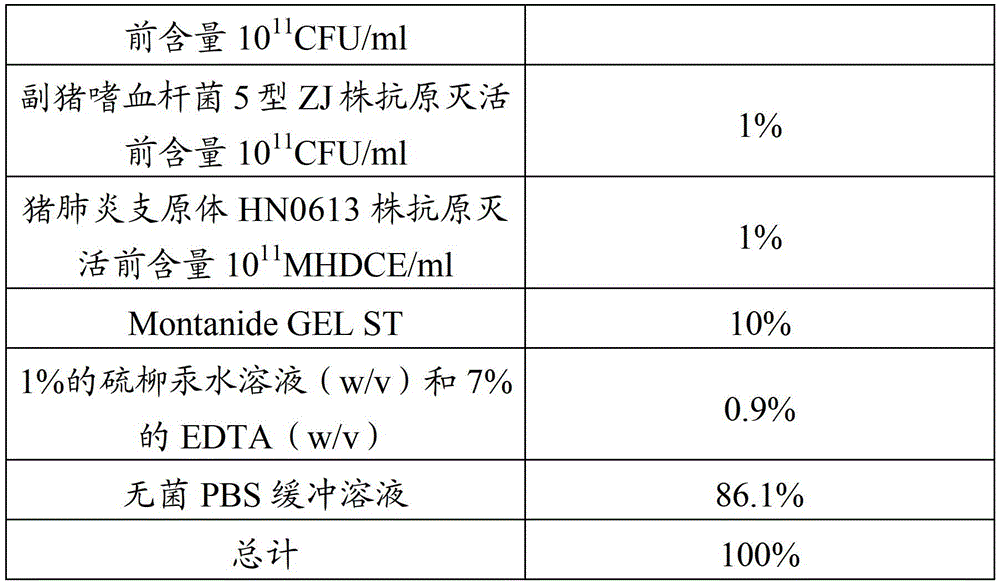
[0060] Haemophilus parasuis disease and Mycoplasma hyopneumoniae inactivated vaccine, composed of inactivated, concentrated and security-checked Haemophilus parasuis serotype 4 JS strain, type 5 ZJ strain antigen and Mycoplasma hyopneumoniae HN0613 strain antigen, and Montanide GEL The ST adjuvant is mixed and prepared, the mixed antigen accounts for 75% to 90% of the total volume of the vaccine, and the adjuvant content is 10% to 25%.
[0061] 1. Preparation of Haemophilus parasuis and Mycoplasma hyopneumoniae seeds
[0062] 1.1 Preparation of Haemophilus parasuis seeds
[0063] Haemophilus parasuis serotype 4 JS strain and type 5 ZJ strain freeze-dried strains were streaked and inoculated on the preferred Haemophilus parasuis medium (MHPs) plate containing agar powder in our laboratory, and placed at 37°C Cultivate for 18-24 hours, select the colonies that meet the r...
Embodiment 3
[0094] Example 3 Porcine Reproductive and Respiratory Syndrome Live Vaccine-Mycoplasma hyopneumoniae, Haemophilus parasuis Inactivated Vaccine Correlation Test
[0095] 1. Physical property test, sterility test
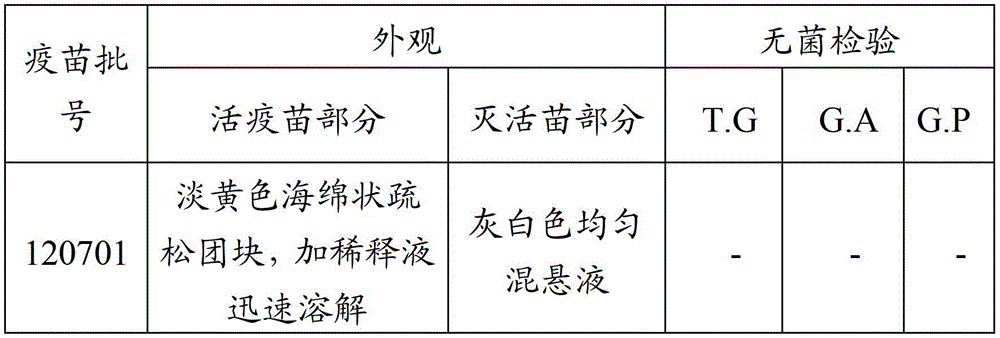
[0096] One batch of porcine reproductive and respiratory syndrome live vaccine - Mycoplasma hyopneumoniae and Haemophilus parasuis inactivated vaccine (batch number 120701), passed the physical property test and sterility test. The detailed results are shown in Table 2:
[0097] Table 2 Vaccine physical properties test and sterility test
[0098]
[0099] Note: T.G means thioglycolate medium, G.A means casease agar medium, G.P glucose-peptone medium; "-" means sterile growth.
[0100] 2 safety test
[0101] Use 5 piglets negative for porcine reproductive and respiratory syndrome virus, Haemophilus parasuis antigen, and Mycoplasma hyopneumoniae antibody at the age of 3 to 4 weeks, and inoculate each muscle with 10 parts of live vaccine diluted with 2 parts of inac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com