A vaccine adjuvant composition for treating or preventing porcine infectious diseases
A vaccine composition and a technology for infectious diseases, which are used in the field of vaccine adjuvant compositions for treating or preventing infectious diseases in pigs, can solve problems such as the limited number of adjuvants, reduce usage, improve immune efficacy, and improve vaccine immunity. protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the vaccine adjuvant composition containing porcine circovirus antigen and vaccine thereof
[0040] 1. Preparation of porcine circovirus antigen
[0041] 1.1 Preparation of bacteria (virus) species for production
[0042] The preparation of porcine circovirus type 2 SH: virus seed is used MEM medium ( Invitrogen Company) was diluted 10 times, inoculated in PK15 (ATCC, preservation number is CCL-33) cell culture by 5% volume, adsorbed at 37°C for 30 minutes, added D-glucosamine containing 4% calf serum and 2mmol / L MEM cell maintenance solution of hydrochloric acid, cultivated at 37°C for 4 days, freeze-thawed 2-3 times, harvested the virus, and the virus titer was 10 6.5 TCID 50 / ml.
[0043] 1.2 Culture preparation of virus liquid or bacterial liquid
[0044] The preparation of porcine circovirus type 2 SH strain virus liquid: the method of cell culture in roller bottles. For the PK15 cells (purchased from ATCC) covered with a single layer, the cell...
Embodiment 2
[0083] The immune effect contrast of embodiment 2, test example 1-10
[0084] 1. Experimental animals and grouping
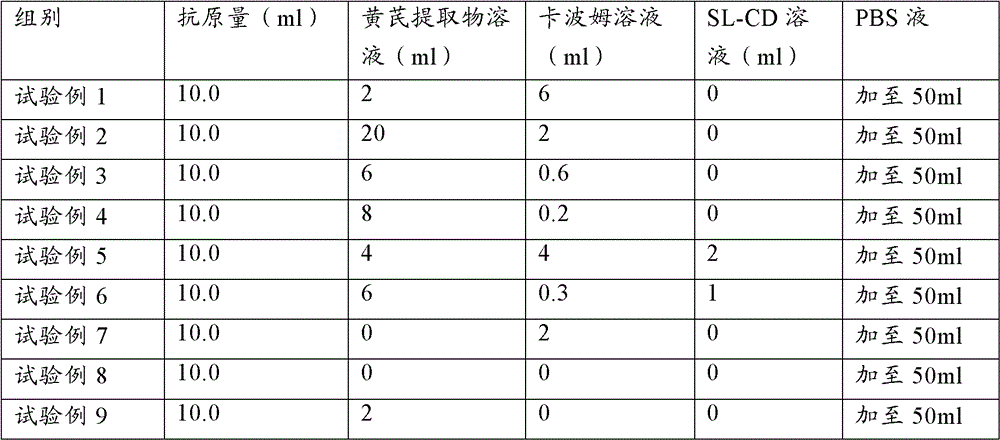
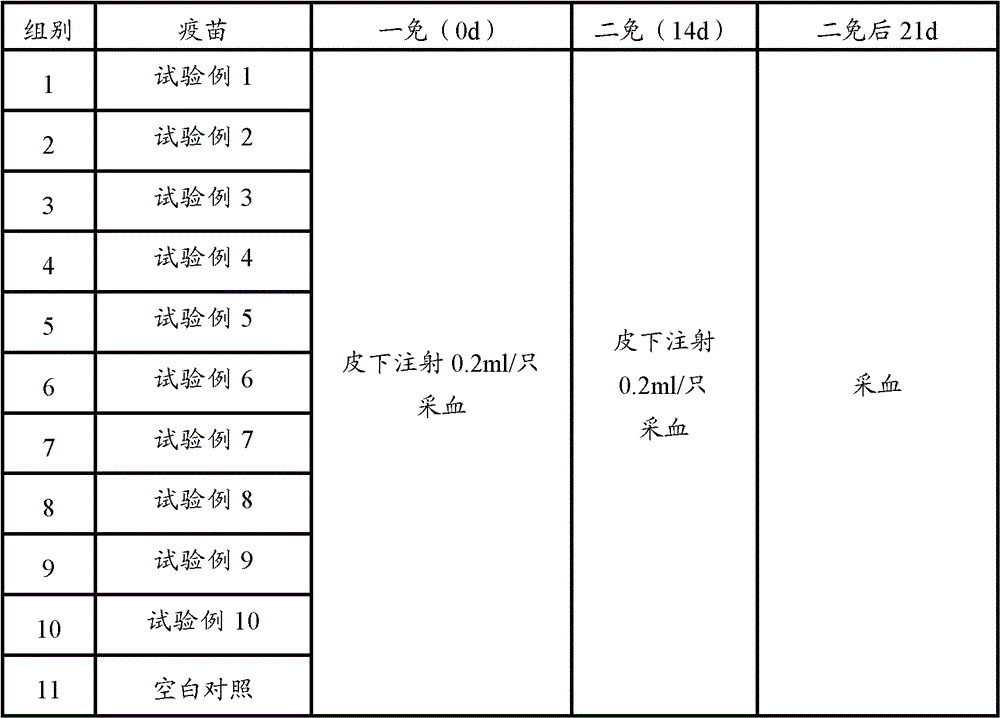
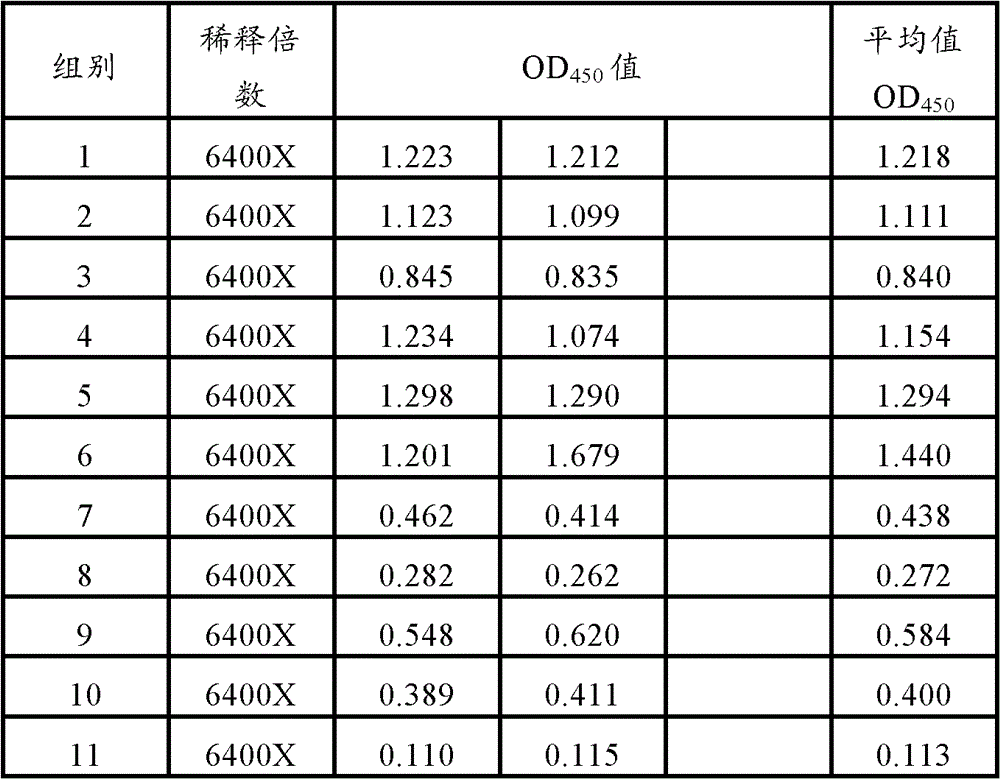
[0085] 60 PCV2 ELISA antibody-negative healthy female clean-grade Balb / c mice aged 5-6 weeks (PCV2 ELISA antibody titer not higher than 1:50) were used and divided into 12 groups, 5 mice in each group. According to Table 1, 0.2ml of each animal was immunized, and the second inoculation was carried out by the same route and dose two weeks later; the blank control was inevitable. The mice in each group were kept in isolation for observation. Blood was collected 3 weeks after the second immunization, the serum was separated, and the PCV2 ELISA antibody titer in the serum was determined. Serum samples from 5 mice were mixed and diluted 6400X, 12800X, and each dilution was repeated for 2 wells
[0086] Table 2 Test grouping and treatment table
[0087]
[0088] 2. Mouse antibody detection
[0089] Use PCV2 ELISA antibody detection method: use PCV2 recombinant...
Embodiment 3
[0113] Embodiment 3, the vaccine adjuvant composition containing swine mycoplasma pneumonia antigen and vaccine thereof
[0114] 1. Preparation of Mycoplasma Pneumonia Antigen
[0115] Strains: Mycoplasma suis pneumonia was selected as strain MR48, and it was preserved in the General Microbiology Center of China Committee for the Collection of Microorganisms on September 19, 2006. The abbreviation of the preservation unit: CGMCC, the preservation number: CGMCCNo.1816, the classification name: pig Mycoplasma pneumoniae, the English name is Mycoplasma hyopneumoniae, address of depository unit: No. 1 courtyard, Beichen West Road, Chaoyang District, Beijing, Institute of Microbiology, Chinese Academy of Sciences 1. Propagation of first-class seeds: get Mycoplasma hyopneumoniae MR48 strain freeze-dried bacterial classification respectively, use Dilute the liquid medium, inoculate it on a solid medium plate, culture it at 37°C for 7-10 days, select a well-growing colony, and use it ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com