Mycoplasma hyopneumoniae and haemophilus parasuis combined inactivated vaccine and application thereof
A technology of Mycoplasma hyopneumoniae and double inactivated vaccine, which is applied in the direction of antibacterial drugs, bacterial antigen components, antibody medical components, etc., can solve the problems of lack of immune cross protection, etc., and achieve good immunogenicity, good safety, and feed remuneration. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Isolation and identification of Mycoplasma hyopneumoniae
[0035] The mycoplasma hyopneumoniae disease material of the present invention comes from the lungs of a 2-month-old nursery pig that had severe pneumonia and dyspnea in a large pig farm in Puyang City, Henan Province in April 2017.
[0036] 1.1 Preparation of medium
[0037] PPLO liquid medium: 10.5 g of PPLO broth powder, 2.5 g of glucose, and 2.5 g of yeast powder, dissolved in 440 mL of ultrapure water, sterilized at 115°C for 15 min, added with 5 mL of MEM medium, 50 mL of horse serum, penicillin 80,000 units, 10 mL of sterile 10% arginine and 500 μL of 1% (w / v) phenol red. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0038] PPLO solid medium: PPLO liquid medium with 1.5% (w / v) agar powder added. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0039] 1.2 Isolation and culture of mycoplasma
...
Embodiment 2
[0053] Example 2: Isolation and identification of bacterial strain HN1553
[0054] 2.1 Culture medium preparation
[0055] TSB liquid medium: Dissolve 30 g of TSB broth powder (Tryptic Soy broth, tryptone soybean broth) in 1000 mL of ultrapure water, sterilize at 115 °C for 15 min, add 50 mL of fetal bovine serum, sterile 1% NAD (Nicotinamide adenine dinucleotide, coenzyme I) 1 mL.
[0056] TSA solid medium: Dissolve 40 g of TSA agar powder (Tryptic Soy Agar, tryptone soybean agar) in 1000 mL of ultrapure water, sterilize at 115 °C for 15 min, add 50 mL of fetal bovine serum, sterile 1% NAD (add as needed ) 1mL; store at 4°C for later use.
[0057] 2.2 Isolation and cultivation of strain HN1553
[0058] In 2018, the applicant collected the heart blood of nursery pigs with typical polyserositis under aseptic conditions, inoculated them on TSA solid medium containing NAD, cultured them in a constant temperature incubator at 37°C for 36 hours, and picked suspected colonies for...
Embodiment 3
[0075] Example 3: Isolation and identification of bacterial strain HN1570
[0076] 3.1 Culture medium preparation
[0077] TSB liquid medium and TSA solid medium were prepared according to the method in Example 2.
[0078] 3.2 Isolation and cultivation of strain HN1570
[0079] In 2017, the applicant collected the heart blood of nursery pigs with typical polyserositis under aseptic conditions, inoculated them on TSA solid medium containing NAD, cultured them in a constant temperature incubator at 37°C for 36 hours, and picked suspected colonies for passage Purification culture.
[0080] 3.3 Identification of strain HN1570
[0081] 3.3.1 Morphological observation and biochemical identification
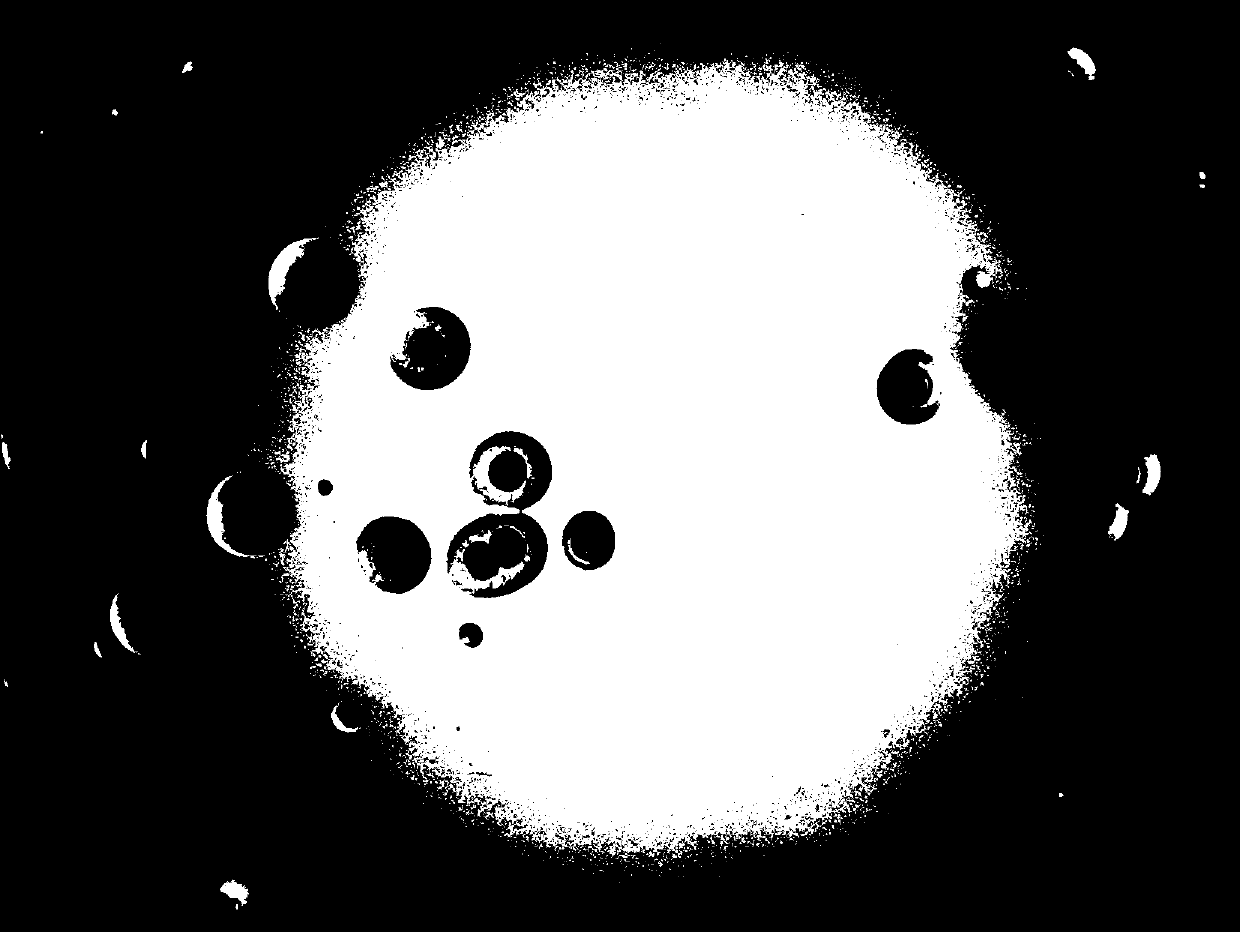
[0082] Observe the colony morphology of Haemophilus parasuis after 24 to 48 hours of subculture of suspected colonies, and grow consistent needle-shaped, colorless, transparent, smooth, moist colonies with a diameter of 1 to 2 mm (see Figure 7 ); at the same time of purification a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com