Vaccine composition, and preparation method and applications thereof
A technology of vaccine composition and antigen, which is applied in the field of vaccine composition, can solve problems such as increased stress response of pig herds, unsatisfactory immune effect of single vaccine, cumbersome operation procedures, etc., and achieves less animal stress and side effects, Effects of Eliminating Maternal Antibody Interference and Simplifying Immunization Procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, preparation of mycoplasma swine pneumonia and Haemophilus parasuis dual inactivated vaccine
[0033] 1. Preparation of Mycoplasma hyopneumoniae antigen
[0034] After unsealing the freeze-dried strains, inoculate the liquid culture medium with 10% inoculum amount, shake and culture at 37°C for 3 to 7 days, and harvest when the pH value drops from 7.5 to 6.8. After pure inspection, they are used as first-class production seeds. Take the first-grade seeds and inoculate the liquid medium with 5% inoculum, shake and culture at 37°C for 3-7 days, harvest when the pH value drops from 7.5 to 6.8, and use them as secondary production seeds after pure inspection. The secondary seeds of Mycoplasma hyopneumoniae HN0613 strain were inoculated in liquid medium at 5% (v / v), cultured with shaking at 37°C for 3 to 7 days, and harvested when the pH value dropped from 7.5 to 6.8.
[0035] 2. Preparation of bacterial liquid of Haemophilus parasuis (type 4 JS strain and type...
Embodiment 2
[0062] Embodiment 2, this study is to evaluate the protective effect of the Mhp-Hps dual inactivated vaccine of trial production in resisting Mhp and Hps attack
[0063] 1. Test material
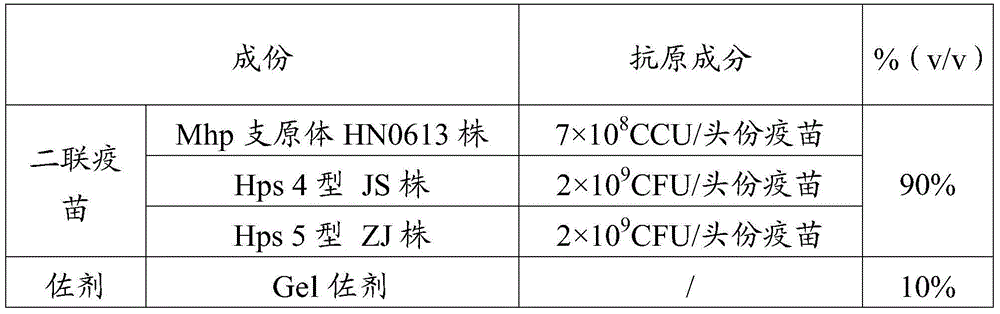
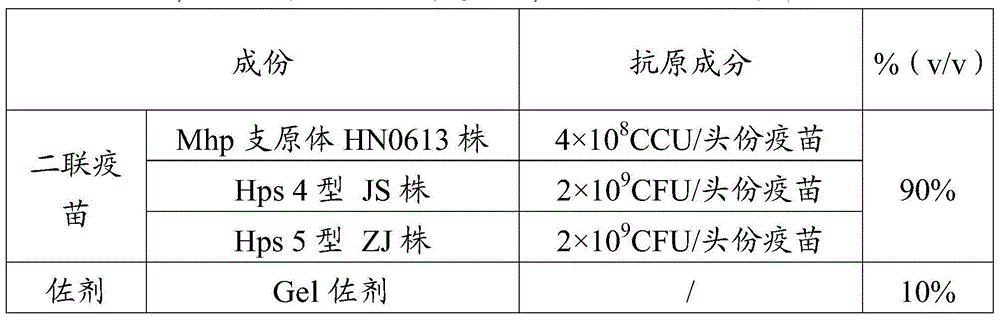
[0064] Vaccine 1 was prepared according to the method in Example 1 (Mhp antigen content was 7×10 8 CCU / toufen, Hps4 type JS strain antigen content is 2×10 9 CFU / portion, Hps5 type ZJ strain antigen content is 2×10 9 CFU / head) and vaccine 2 (Mhp antigen content is 4×10 8 CCU / toufen, Hps4 type JS strain antigen content is 2×10 9 CFU / portion, Hps5 type ZJ strain antigen content is 2×10 9 CFU / head serving).
[0065] 2. Design of animal experiments
[0066] 2.1 Vaccine Immunization
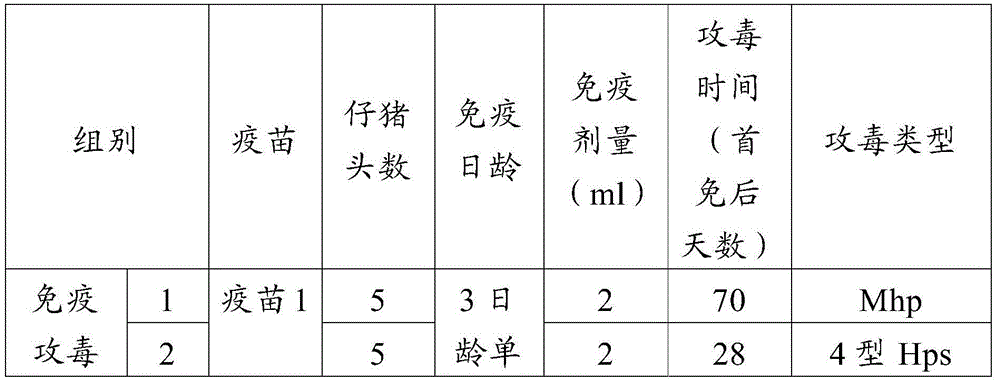
[0067] A total of 110 healthy susceptible piglets aged 0 to 3 days were selected. The specific implementation scheme is shown in Table 3, and the route of vaccination is neck intramuscular injection.
[0068] Table 4 Mhp-Hps dual vaccine immunization groups
[0069]
[0070]
[0071] 2.2 Mycoplasma hyo...
Embodiment 3
[0092] Example 3. This study is to evaluate the influence of maternal antibodies on the trial-manufactured Mhp-Hps dual combination vaccine 1 and the combination of commercially available Mycoplasma hyopneumoniae single vaccine and Haemophilus parasuis inactivated vaccine
[0093] 1. Test material
[0094] Vaccine 1 was prepared according to the method in Example 1 (Mhp antigen content was 7×10 8 CCU / toufen, Hps4 type JS strain antigen content is 2×10 9 CFU / portion, Hps5 type ZJ strain antigen content is 2×10 9 hyopneumoniae single vaccine (Rebex) and Haemophilus parasuis inactivated vaccine (produced by Huazhong Agricultural University, Wuhan Keqian Animal Biological Products Co., Ltd., and China Animal Husbandry Co., Ltd. ).
[0095] 2. Design of animal experiments
[0096] 2.1 Vaccine Immunization
[0097] A total of 55 healthy susceptible piglets aged 0 to 3 days were selected, and the Mhp antibody and Hps antibody monitoring were all positive, suggesting that the pig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com