Pig mycoplasma pneumonia live attenuated vaccine and application thereof
A technology of Mycoplasma suis pneumonia and live attenuated vaccine, applied in bacterial antigen components, microorganisms, respiratory system diseases, etc., can solve the problems of difficult to achieve ideal control, high price, unable to completely prevent wild virus infection and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
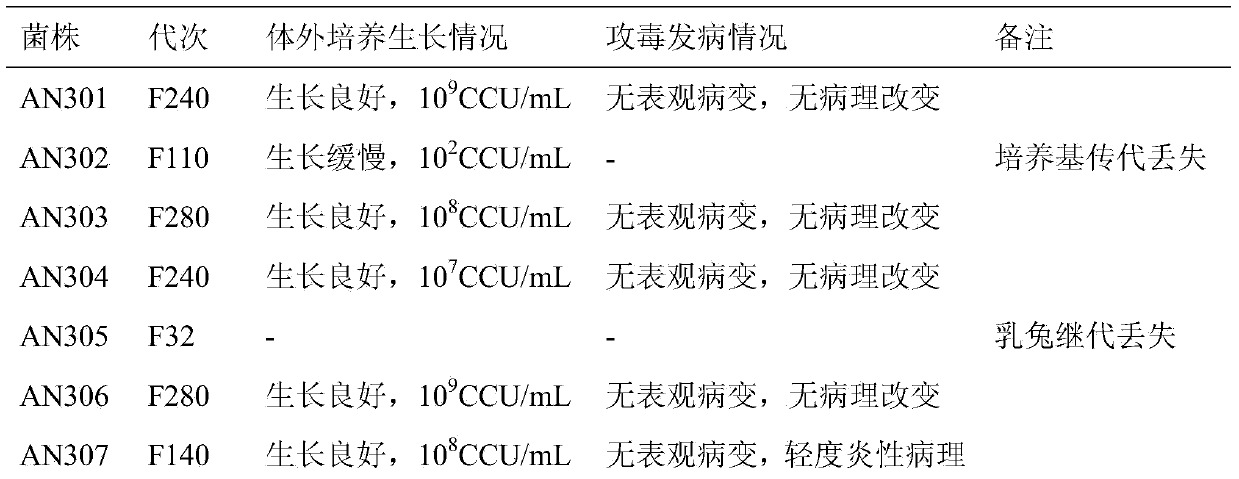
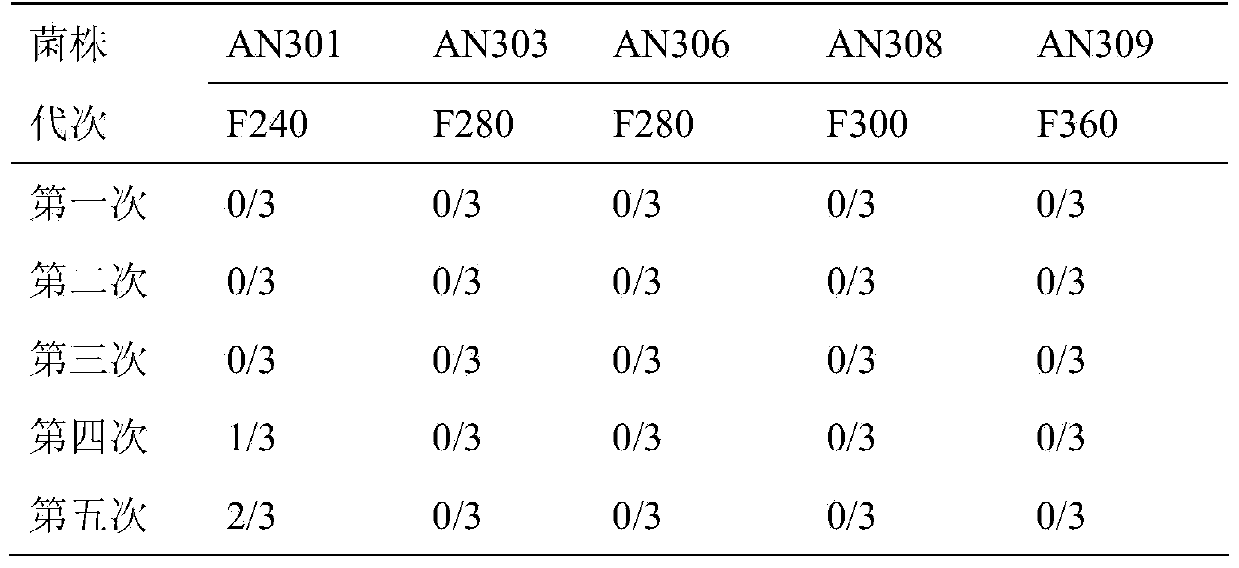
[0024] Embodiment 1: the acquisition of Mycoplasma hyopneumoniae AN306
[0025] Acquisition of virulent strains:
[0026] In 1975, according to the requirements of the Ministry of Agriculture, the Jiangsu Academy of Agricultural Sciences collected typical disease materials of mycoplasma pneumonia from more than 20 provincial agricultural universities and national and provincial academies of agricultural sciences to screen the virulent pathogenic strains of mycoplasma hyopneumoniae. A total of more than 200 samples of suspected mycoplasma pneumonia pneumonia lung disease were collected from all over the country, and 10 samples with typical mycoplasma hyopneumoniae infection and no obvious other pathogenic infection were screened out, and they were stored as virulent tissue poisons of mycoplasma hyopneumoniae. . Simultaneously, the animal challenge pathogenesis test and the continuous passage attenuation test were carried out.
[0027] The selected 10 highly virulent tissue vi...
Embodiment 2
[0047] Embodiment 2: the cultivation of Mycoplasma hyopneumoniae attenuated strain AN306 strain
[0048] Preparation of KM2 liquid medium (according to 2050mL): Eagles’ solution 1000mL, hydrolyzed milk protein 10g, porcine serum 400mL, fresh yeast extract 20mL, Dulbecco’s phosphate buffer 600mL, penicillin 4 million units, 0.4% phenol red 3.5mL, Use 10g / L NaOH to adjust the pH value to 7.4-7.6.
[0049] Take 1 freeze-dried strain of Mycoplasma hyopneumoniae attenuated strain AN306, dissolve it in 0.5mL medium, inoculate 0.5mL in 4.5mL medium, culture in a closed penicillin bottle at 37°C, when the color of the medium changes from red to yellow Harvest the culture in time, inoculate it into fresh medium at a ratio of 1:10 by volume to expand the culture, harvest the culture in time when the color of the medium changes from red to yellow, expand the culture to 5000mL and harvest the strain accordingly.
[0050] Prepare a lyoprotectant, the formula is 1.5 g of gelatin, 12.5 g of...
Embodiment 3
[0052] Example 3: Immunoprotective evaluation of intrapulmonary injection immunized live attenuated Mycoplasma pneumoniae vaccine AN306 strain and comparison with other commercialized live vaccines and inactivated vaccines
[0053] Vaccine preparation:
[0054] Freeze-dried vaccine of AN306 strain of mycoplasma pneumonia live attenuated vaccine, dissolved in sterile phosphate buffer solution, prepared vaccine solution so that the titer of the live vaccine was 2×10 5 CCU / mL. Inject 0.5 mL into the lungs of each animal, that is, 10 5 CCU / head.
[0055] Commercialized live vaccines: In this experiment, only two live vaccines against Mycoplasma pneumoniae in the market were used as control live vaccines. (1) The live vaccine of Mycoplasma suis pneumonia produced by Jilin Zhengye Biological Products Co., Ltd. is chicken embryo yolk sac tissue containing the attenuated strain of Mycoplasma hyopneumoniae, hereinafter referred to as commercial vaccine 1, and is operated according t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com