Insoluble medicine gel composition and preparation method for same
A technology for insoluble drugs and gel compositions, which is applied in the field of insoluble drug gel compositions and their preparation, temperature-sensitive gel compositions containing insoluble drug nanocrystals and their preparations, and can solve the problems of poor physical stability and temperature-sensitive gel loading. Low drug dosage, short drug release time, etc., to achieve the effect of improving stability, increasing drug loading, and avoiding deposition problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
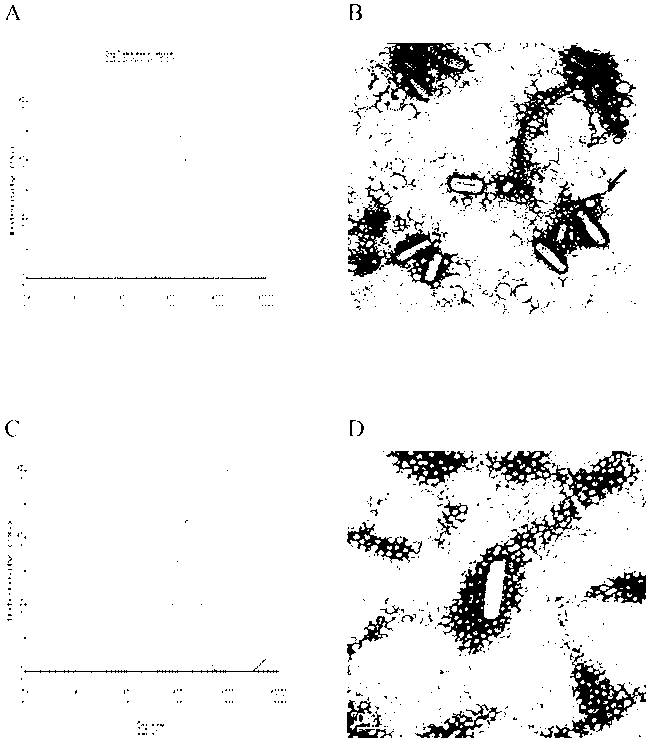
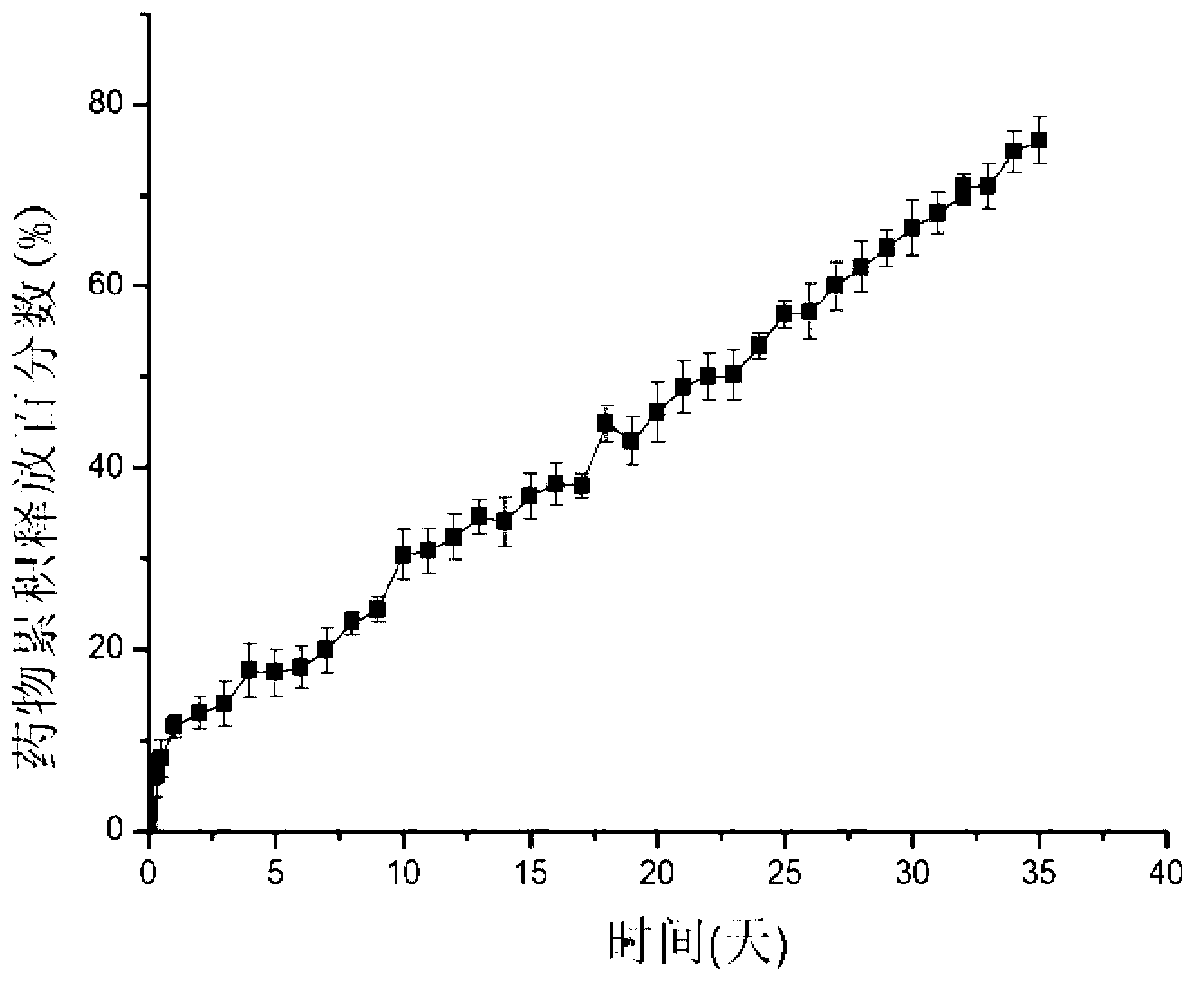
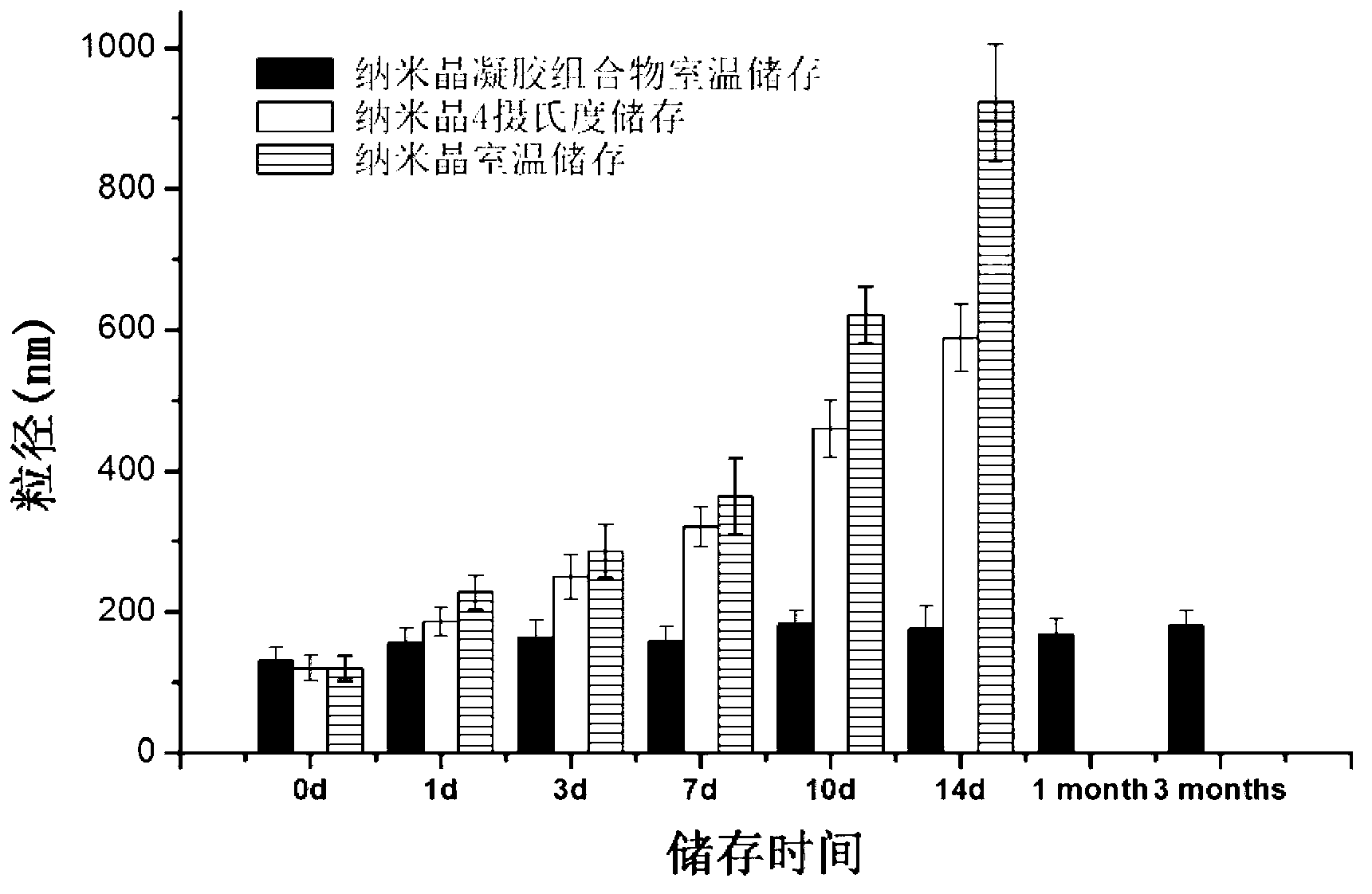
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of paclitaxel nanocrystalline gel composition
[0046] 1. Preparation of drug nanocrystals: Weigh 32 mg of paclitaxel and 160 mg of pluronic F127 respectively, add the drug and surface stabilizer to 4 ml of chloroform, dissolve them completely by ultrasonication, and evaporate the organic matter by nitrogen blowing at a constant flow rate. solvent, and placed in a vacuum oven at 25°C for 12h to remove residual organic solvent. Add 8ml of purified water to the above dried paclitaxel and Pluronic F127 film, hydrate for 40 minutes, vortex for 10 minutes, and sonicate for 10-15 minutes to obtain paclitaxel nanocrystal suspension.
[0047] 2. Preparation of the drug nanocrystal gel composition: Weigh 2 g of Pluronic F127, directly add it to the suspension of paclitaxel nanocrystals, and stir magnetically in an ice bath for more than 6 hours to obtain the combination of paclitaxel drug nanocrystal gel things.
Embodiment 2
[0048] Embodiment 2, the preparation of docetaxel nanocrystalline gel composition
[0049] 1. Preparation of drug nanocrystals: Weigh 32 mg of docetaxel and 160 mg of polyethylene glycol vitamin E succinic acid (TPGS) respectively, add the drug and surface stabilizer to 3 ml of dichloromethane, and ultrasonically dissolve them completely , dried by rotary evaporation under reduced pressure to remove the organic solvent, and placed in a vacuum oven at 25° C. for 12 hours to remove the residual organic solvent. Add 7.5ml of purified water to the above dried docetaxel and TPGS film, hydrate for 40 minutes, vortex for 10 minutes, and sonicate for 10-15 minutes to obtain a suspension of docetaxel nanocrystals.
[0050] 2. Preparation of pharmaceutical nanocrystalline gel composition: Weigh 2.5 g of polycaprolactone-polyethylene glycol-polycaprolactone and directly add it to the suspension of docetaxel nanocrystals, and stir magnetically in an ice bath for 6 hours As above, the com...
Embodiment 3
[0051] Embodiment 3, preparation of docetaxel-paclitaxel nanocrystalline gel composition
[0052] 1. Preparation of docetaxel drug nanocrystals: Weigh 16 mg of docetaxel and 160 mg of Tween 80 respectively, add the drug and surface stabilizer to 5 ml of absolute ethanol, and ultrasonically dissolve it completely. The organic solvent was evaporated by nitrogen blowing at a flow rate, and placed in vacuum drying at 25° C. for 12 h to remove residual organic solvent. Add 5ml of purified water to the above dried docetaxel and Tween 80 film, hydrate for 40 minutes, vortex for 10 minutes, and sonicate for 10-15 minutes to obtain a suspension of docetaxel nanocrystals.
[0053] 2. Preparation of paclitaxel drug nanocrystals: Weigh 16 mg of paclitaxel and 160 mg of surface stabilizer Pluronic F127, respectively, add the drug and surface stabilizer to 4 ml of absolute ethanol, dissolve them completely by ultrasonication, and pass through at a constant flow rate Nitrogen was blown to d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com