Self asembled precusor liposome containing camptothecin kind medicine and its preparation method
A proliposome and self-assembly technology, which is applied in drug combination, liposome delivery, medical preparations containing active ingredients, etc., can solve the problem of reduced drug encapsulation rate, poor stability of preparation quality, complicated preparation process, etc. problem, to achieve the effect of stable and controllable quality, stable product quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Prescription: 10-Hydroxycamptothecin 1g
[0053] Soy Lecithin 180g
[0054] Poloxamer 188 (trade name F-68) 5g
[0055] Benzyl alcohol 250g
[0056] Add absolute ethanol to 1000ml
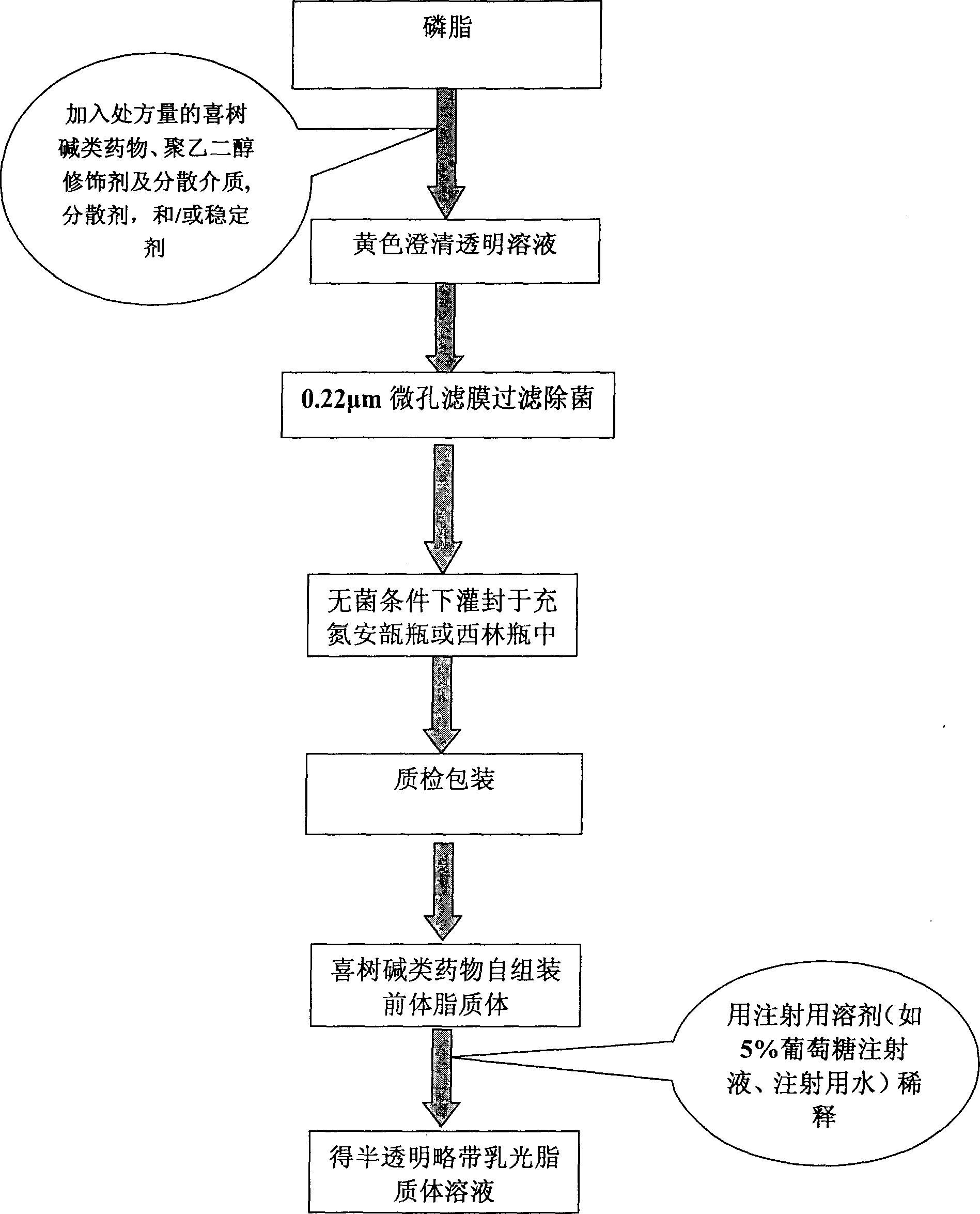
[0057] Preparation process: according to figure 1 Dissolve the prescribed amount of soybean lecithin in an appropriate amount of ethanol, then add the prescribed amount of drug, poloxamer 188, benzyl alcohol and the remaining amount of ethanol. After the drug and other components are completely dispersed or dissolved, After filtering and sterilizing with a 0.22 μm microporous membrane, the filtrate is filled and sealed in a nitrogen-filled ampoule or a vial under aseptic conditions to obtain the 10-hydroxycamptothecin self-assembled proliposome.
[0058] Take the 10-hydroxycamptothecin self-assembled proliposome according to the clinical dose before use and add it to 150 times the amount (v / v) of 5% glucose solution to dilute and disperse, and quickly form 10-hyd...
Embodiment 2
[0060] Prescription: Camptothecin 1g
[0061] Egg phospholipids 200g
[0062] Tween 80 5g
[0063] n-Butanol 150g
[0064] Vitamin E 2g
[0065] Macrogol 400 up to 1000ml
[0066] Preparation process: dissolve the prescribed amount of egg phospholipids in an appropriate amount of polyethylene glycol 400, then add the prescribed amount of medicine, Tween 80, n-butanol, vitamin E and the remaining amount of polyethylene glycol 400, and wait for the medicine and After all the other components are dispersed or dissolved, the 0.22μm microporous membrane is filtered to sterilize, and the filtrate is filled and sealed in nitrogen-filled ampoules or vials under sterile conditions to obtain camptothecin self-assembled proliposomes .
[0067] Take the camptothecin self-assembled proliposome according to the clinical dosage before use and add it to 200 times the amount (v / v) of 5% glucose solution to dilute and disperse, and then quickly form a camptoth...
Embodiment 3
[0069] Prescription: 10-Hydroxycamptothecin 0.8g
[0070] Hydrogenated Phospholipids 200g
[0071] Polyoxyethylene hydrogenated castor oil (Cremophor RH40) 2g
[0072] Benzyl alcohol 120g
[0073] Deoxycholic acid 0.8g
[0074] Propylene glycol 200g
[0075] Add absolute ethanol to 1000ml
[0076] Preparation process: dissolve the prescribed amount of hydrogenated phospholipids in an appropriate amount of ethanol, then add the prescribed amount of drugs, polyoxyethylene hydrogenated castor oil Cremophor RH40, benzyl alcohol, propylene glycol, deoxycholic acid and the remaining amount of ethanol, and wait for the drug and After all other components are dispersed or dissolved, 0.22μm microporous membrane is filtered to sterilize, and the filtrate is filled and sealed in nitrogen-filled ampoules or vials under sterile conditions to obtain the self-assembled precursor of 10-hydroxycamptothecin Liposomes.
[0077] Take the 10-hydroxycamptot...
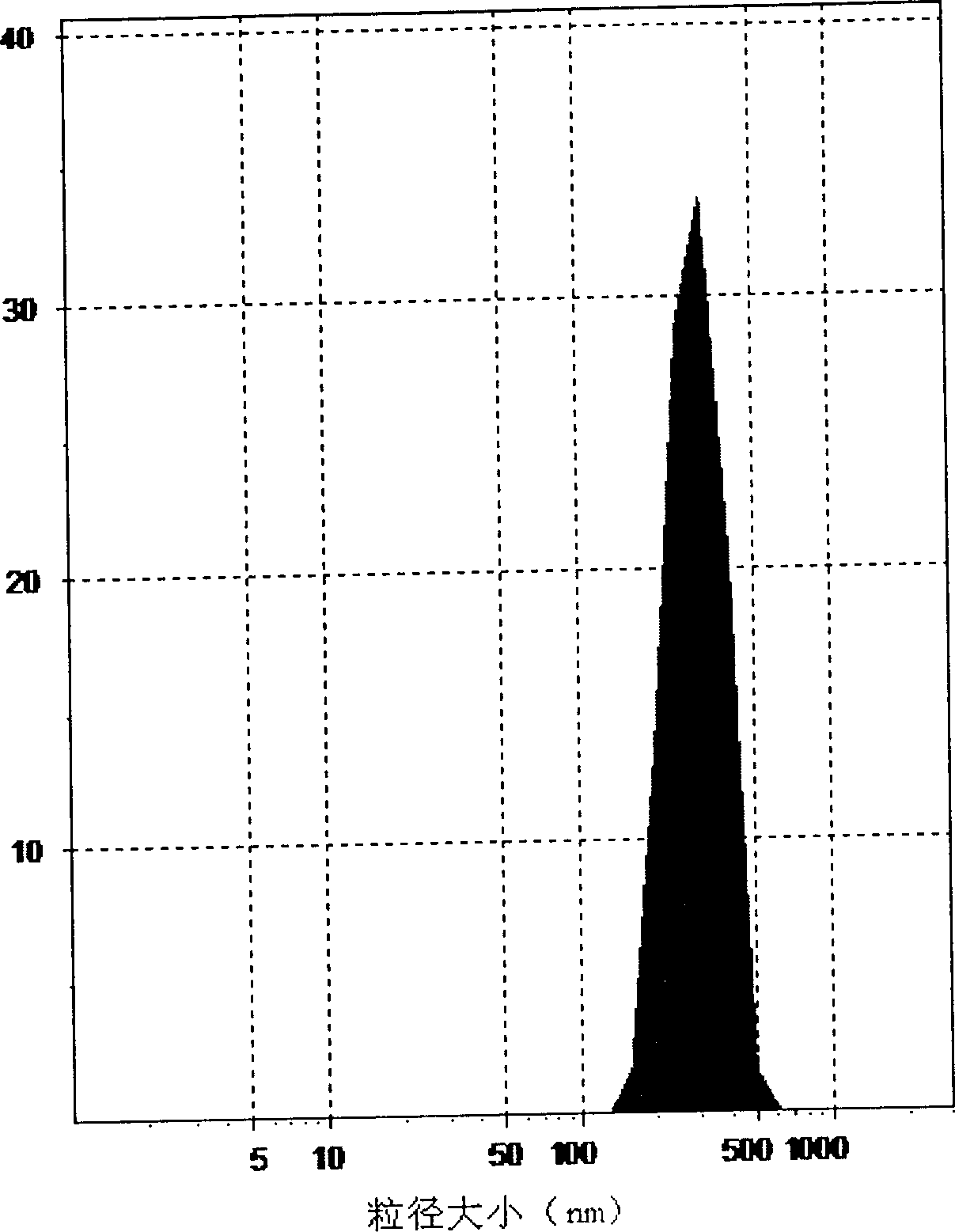
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com