Self assembled precusor liposome containing hard soluble medicine and its preparation method
A technology of proliposomes and insoluble drugs, applied in liposome delivery, medical preparations of non-active ingredients, pharmaceutical formulations, etc., can solve incomplete local dissolution and decreased encapsulation efficiency of drug-containing liposomes , drug encapsulation rate reduction and other issues, to achieve the effect of solving high-efficiency industrial production, safe and easy to purchase, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] prescription:
[0068] Paclitaxel 0.6g
[0069] Soy Lecithin 37.5g
[0070] Polyoxyethylene hydrogenated castor oil (Cremophor RH40) 18.75g
[0071] Cholesterol 2.5g
[0072] Ethanol to 100ml
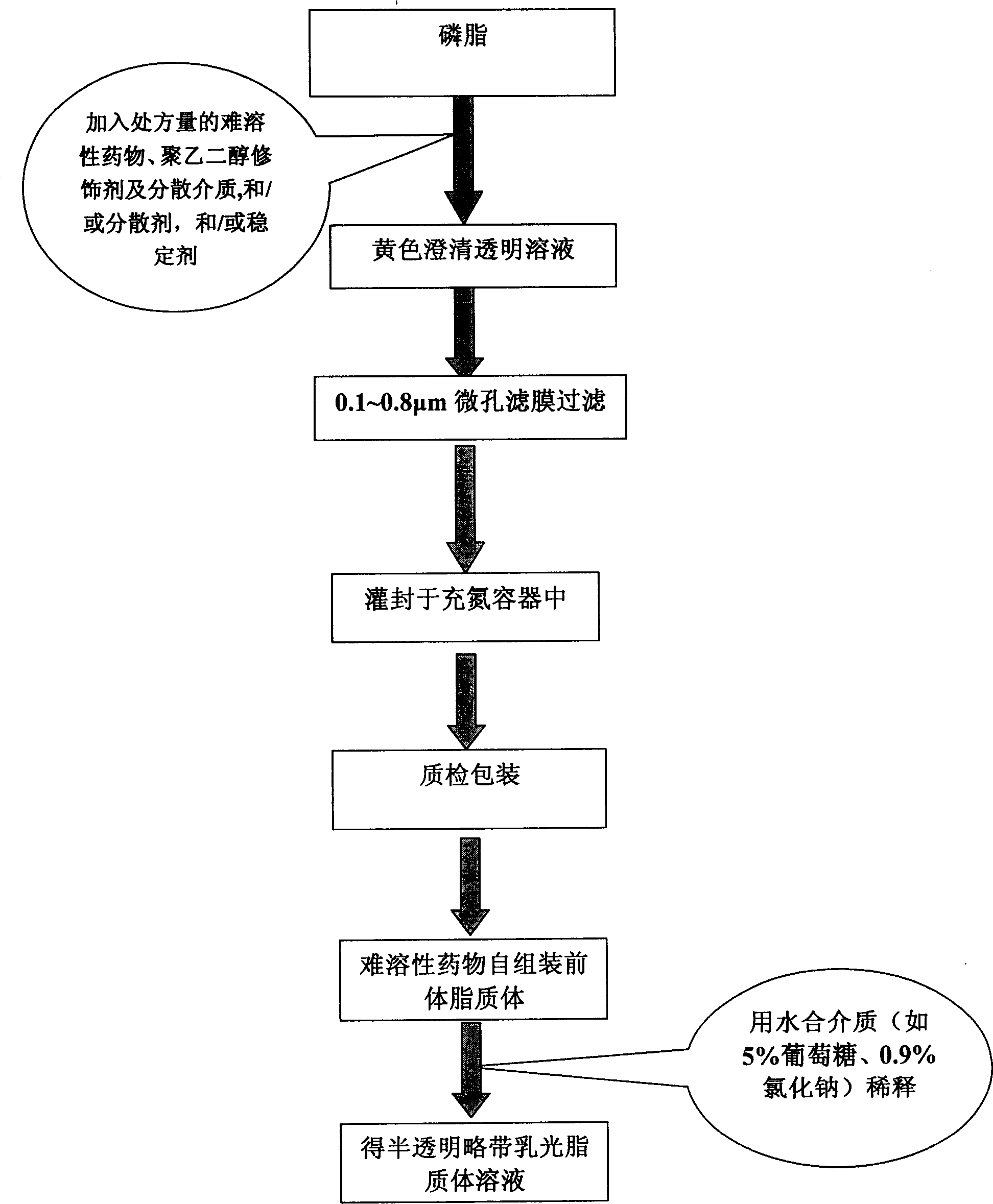
[0073] Preparation process: according to figure 1 The process flow chart shown dissolves the prescribed amount of soybean lecithin in an appropriate amount of ethanol, and then adds the prescribed amount of medicine, polyoxyethylene hydrogenated castor oil Cremophor RH40, cholesterol and the remaining amount of ethanol, after the medicine and other components are completely dissolved , 0.22μm microporous membrane filtration sterilization, under sterile conditions, the filtrate can be sealed in nitrogen-filled ampoules or vials.
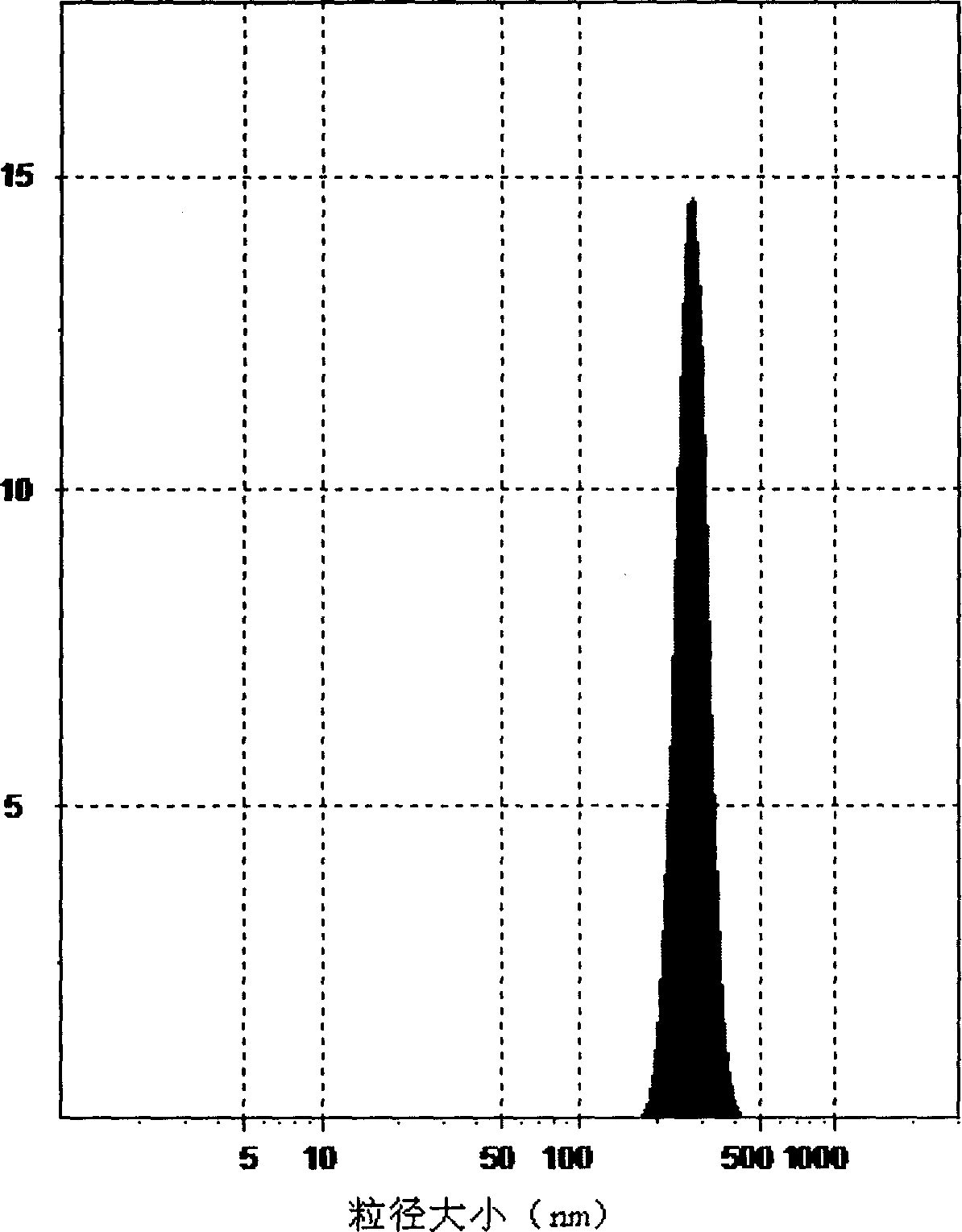
[0074] Adding the prepared paclitaxel self-assembled proliposomes to 50 times the amount (v / v) of 5% glucose injection can quickly form a paclitaxel liposome solution with an average particle size of 95 %.
Embodiment 2
[0076] Prescription: Nimodipine 1.0g
[0077] Egg phospholipids 16.7g
[0078] Tween 80 10.7g
[0079]Polyoxyethylene castor oil (Cremophor EL) 6.0g
[0080] Ethanol up to 50ml
[0081] Preparation process: dissolve the prescribed amount of egg phospholipids in an appropriate amount of ethanol, then add the prescribed amount of nimodipine, Tween 80, polyoxyethylene castor oil Cremophor EL and the remaining amount of ethanol, and wait until the drug and other components are completely dispersed Or after dissolving, filter and sterilize with a 0.22 μm microporous membrane, fill and seal the filtrate in a nitrogen-filled ampoule under aseptic conditions to obtain the nimodipine self-assembled proliposome.
[0082] After adding the prepared nimodipine self-assembled proliposome to 100 times the amount (v / v) of glucose sodium chloride solution, the nimodipine liposome solution can be formed rapidly, with an average particle diameter 90%.
Embodiment 3
[0084] Prescription: Curcuma Oil 1ml
[0085] Soy Lecithin 10g
[0086] Polyoxyethylene hydrogenated castor oil (Cremophor RH60) 3.7g
[0087] Ethanol to 25ml
[0088] Preparation process: dissolve the prescribed amount of soybean lecithin in an appropriate amount of ethanol, then add the prescribed amount of zedoary oil, polyoxyethylene hydrogenated castor oil Cremophor RH60 and the remaining amount of ethanol, after the drug and other components are completely dispersed or dissolved, The 0.22 μm microporous membrane was filtered and sterilized, and the filtrate was filled and sealed in a nitrogen-filled ampoule under aseptic conditions to obtain the self-assembled proliposome of zedoary oil.
[0089] The prepared zedoary oil self-assembled proliposome is added to 25 times the amount (v / v) of physiological saline, and the zedoary oil liposome solution can be rapidly formed, with an average particle size of 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com