Mycoplasma hyopneumoniae indirect ELISA antibody detection kit and application
A technology of mycoplasma hyopneumoniae and DNA sequence, which is applied in the detection field of animal virology and zoonosis, and achieves the effect of short detection time, broad market prospect and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
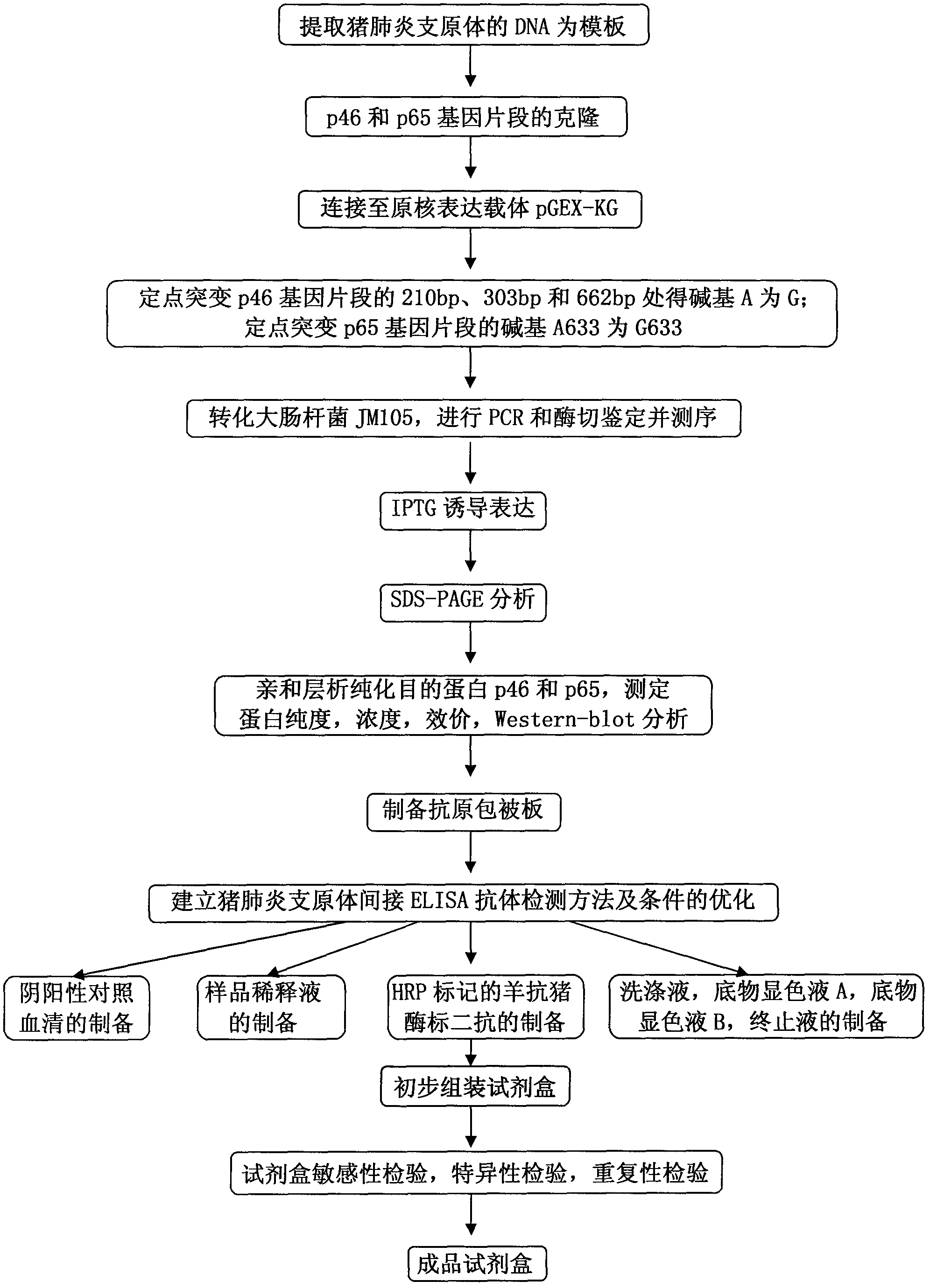
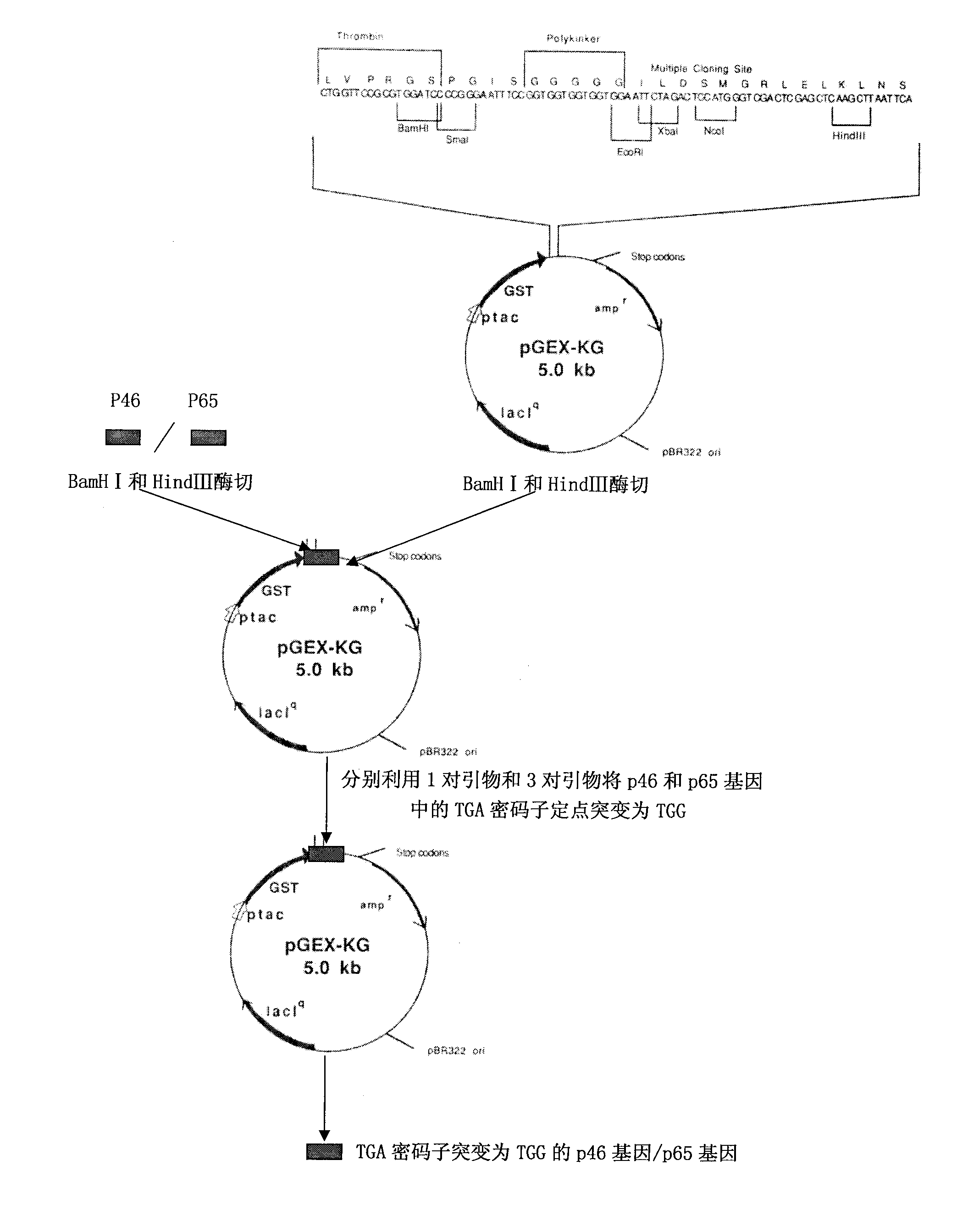
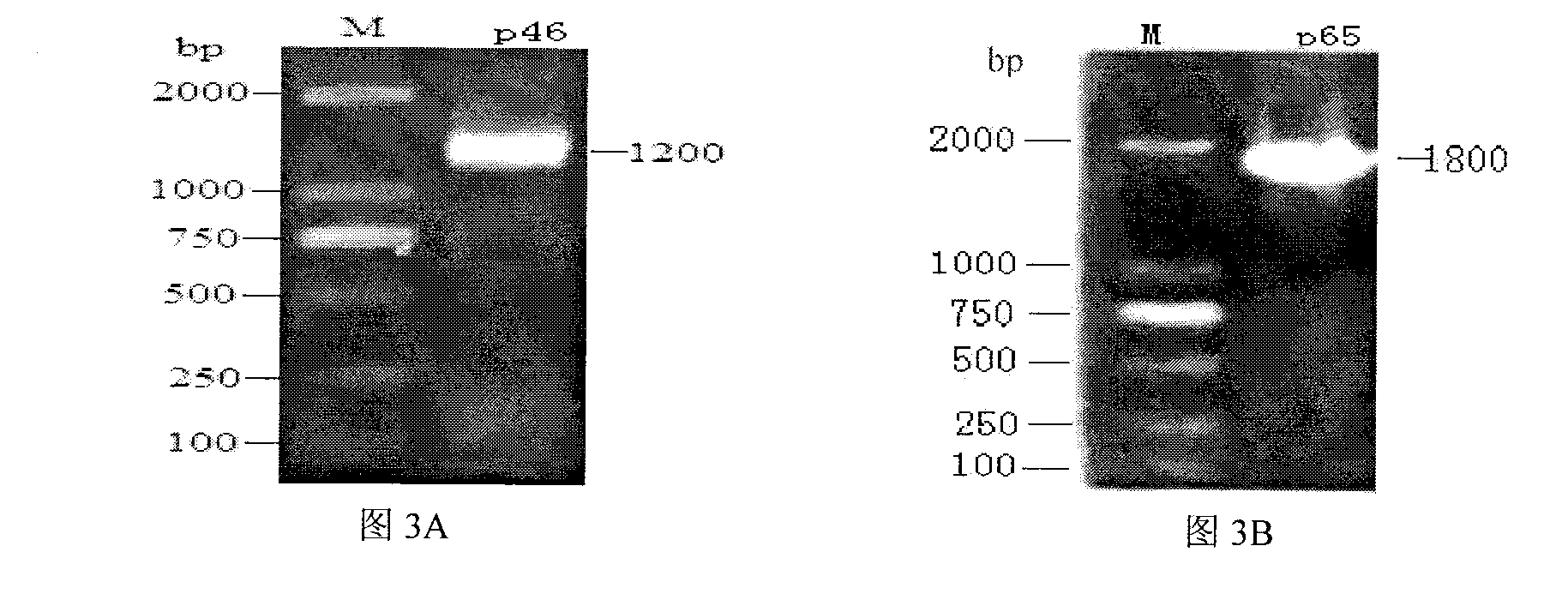
[0067] Example 1 Cloning of gene fragments of Mycoplasma hyopneumoniae p46 protein and p65 protein
[0068] 1. For the cloning method of the p46 and p65 protein gene fragments of Mycoplasma hyopneumoniae, see figure 2 shown.
[0069] The primers required to prepare the p46 gene fragment capable of prokaryotic expression are listed in Table 1.
[0070] Table 1 prepares the primers required for the p46 gene fragment capable of prokaryotic expression
[0071]
[0072] Note: The underlined part of the primers shown in Table 1 is the restriction site.
[0073] Table 2 prepares the primers required for the p65 gene fragment capable of prokaryotic expression
[0074]
[0075] Note: The underlined part of the primers shown in Table 2 is the restriction site.
[0076] 2. Preparation of p46 and p65 gene fragments capable of prokaryotic expression
[0077]Genomic DNA was extracted from the live vaccine of Mycoplasma hyopneumoniae (purchased from Nanjing Tianbang Biotechnology...
Embodiment 2
[0084] Example 2 Identification of recombinant strains pGEX-KG-46 and pGEX-KG-65 expressing p46 and p65 fusion proteins
[0085] Inoculate the strain with the correct mutation into the LB liquid medium containing 100mg / ml ampicillin at a ratio of 1:100 (volume ratio), shake and culture at 200r / min at 37°C for 3 hours, then inoculate again at a volume ratio of 1:1000 Add isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.8mmol / L, and induce expression for 4 hours. Collect the bacteria and use 10mL Buffer A (recipe: weigh 6.057g Tris base, 0.1861g disodium edetate, 2.922g sodium chloride, 50mL glycerin, dissolve in deionized water and dilute to 1000mL, and use 0.22μm Store at 2-8°C after filtering through a filter membrane, and resuspend in 0.5mM DTT when used. After repeated freezing and thawing three times, perform ultrasonic crushing (UP 200S ultrasonic processor-manufactured by Dr. Hielscher, Germany, power: 200W, amplitude: 60%, operating frequency: 2...
Embodiment 3
[0086] Example 3 Preparation and testing of antigen
[0087] 1. Purification and inspection of p46 and p65 fusion proteins
[0088] GST-46 and GST-65 expressed in soluble form were purified by agarose-4B affinity layer suction column: add the supernatant to a centrifuge tube containing about 1 mL of GST-4B stock solution, place on a horizontal shaker at room temperature or After shaking slowly for 1 h under lower conditions, centrifuge at 2100 r / min for 5 min, transfer the supernatant to another centrifuge tube, add at least 10 times the volume of PBS with pH 7.4 (recipe: weigh 8.18 g sodium chloride, 0.20 g Potassium chloride, 1.42g disodium hydrogen phosphate, 0.245g potassium dihydrogen phosphate, dissolve in deionized water, adjust pH to 7.4 with concentrated hydrochloric acid, dilute to 1000mL with deionized water, then filter with 0.22μm filter membrane Store at room temperature.) Shake on a shaker for about 10 minutes, centrifuge at 2100r / min for 5 minutes, wash twice,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com