Method for in vitro dissolution of minocycline hydrochloride sustained-release tablet
A technology of minocycline hydrochloride and its determination method, which is applied in the field of in vitro dissolution determination of minocycline hydrochloride sustained-release tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Tablet specification 55mg (100 small test):
[0039]Get two batches of the same specification (55mg) small test piece and each 6 tablets of the reference preparation of the same specification, first take 500mL solution 1 as the dissolution medium, then use 900mL solution 2 as the dissolution medium, and operate under the other reference [0017].
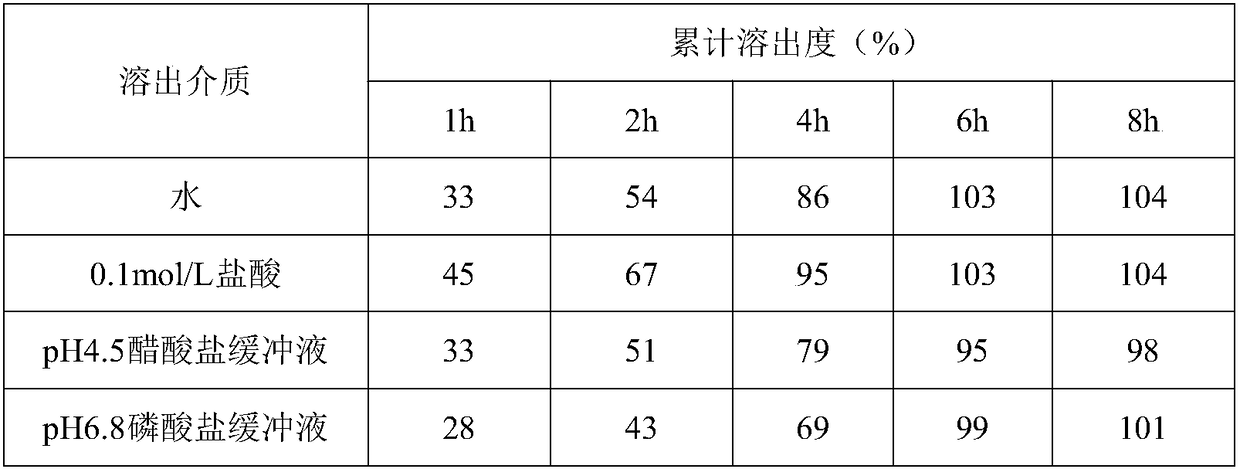
[0040] The same dissolution conditions of table 3 affect the dissolution profiles of different small test batches 1
[0041]
[0042]
[0043] Test results: Under the same conditions, the dissolution of different batches did not change much, and the fitting degree of preparation 1 was higher.
Embodiment 2
[0045] Tablet specification 55mg (1000 pilot test):
[0046] Get two batches of each 6 pieces of same specification (55mg) middle test sheet, carry out stripping with reference to [0017] stripping method, obtain cumulative release by external standard method.
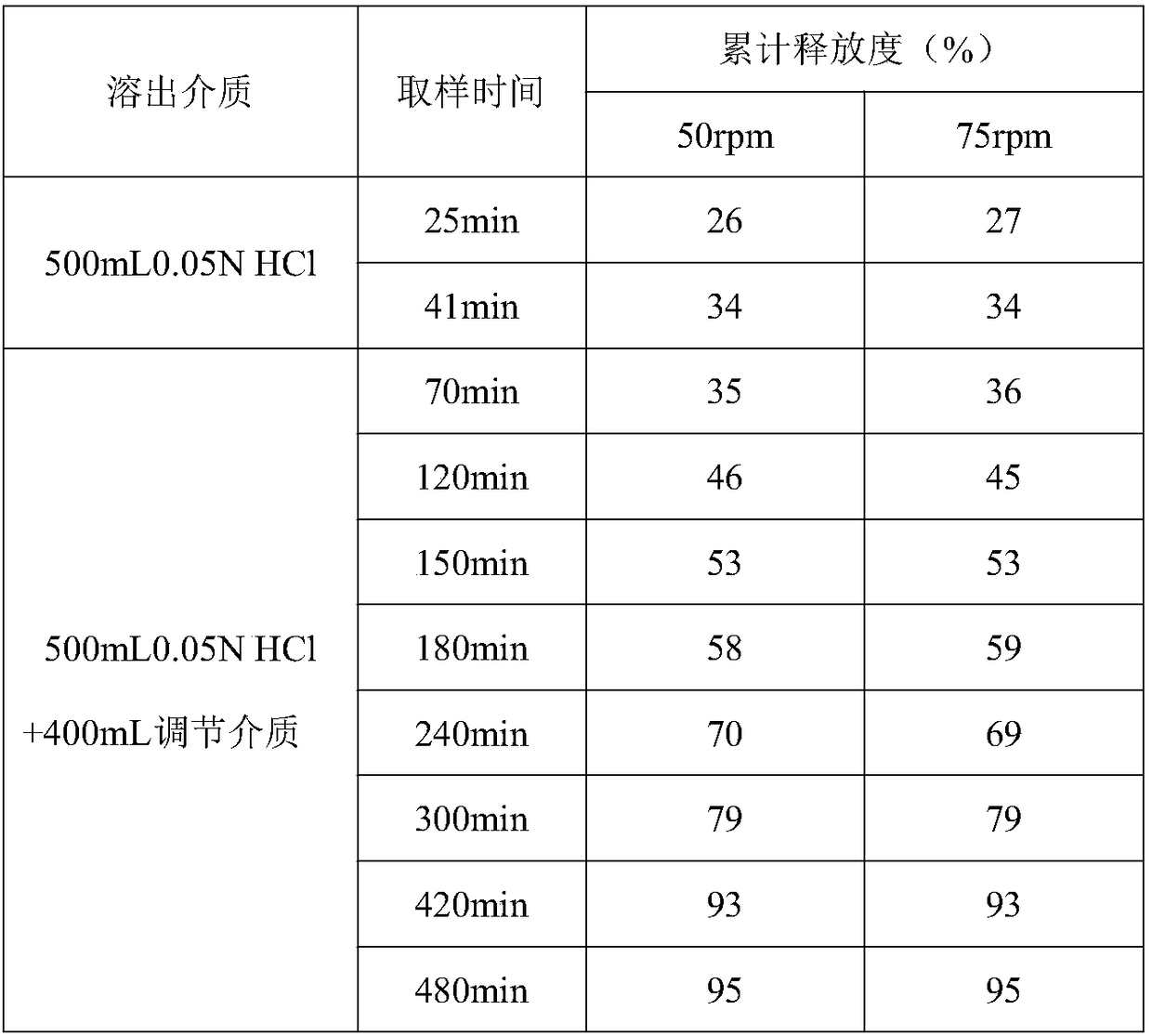
[0047] The same dissolution condition of table 4 is to the impact of different small test batch dissolution curves 2
[0048]
[0049] Test results: Under the same conditions, the dissolution of different batches did not change much, and the fitting degree of preparation 3 was higher.
Embodiment 3
[0051] Tablet specification 55mg (10,000 enlarged batches):
[0052] Get two batches of same specification (55mg) each 6 that amplify large scale sheet, carry out stripping with reference to [0017] stripping method, obtain cumulative release by external standard method.
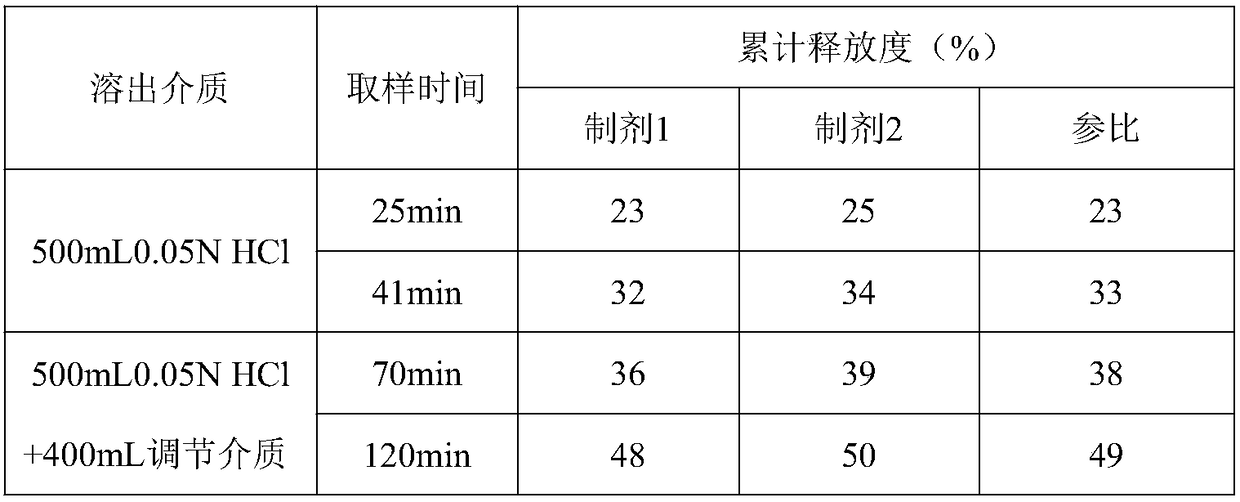
[0053] The same dissolution condition of table 5 is to the impact of different small test batch dissolution curves 3
[0054]
[0055] Test results: Under the same conditions, the dissolution of different batches did not change much, and the fitting degree of preparation 6 was higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com