Minocycline hydrochloride sustained release tablet and preparation method thereof
A technology of minocycline hydrochloride and sustained-release tablets, which is applied to tetracycline active ingredients, pharmaceutical formulations, pill delivery, etc., to achieve the effects of increasing stability, reducing production costs, and shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
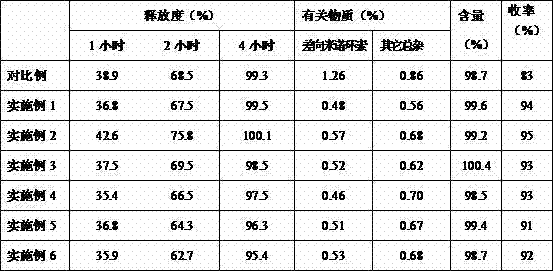
Examples
Embodiment 1
[0053] Embodiment 1: a kind of minocycline hydrochloride slow-release tablet, its prescription is as follows:
[0054] Minocycline hydrochloride 45.0g (as minocycline)
[0055] Hypromellose (E50LV) 95.0g
[0056] Lactose 230.0g
[0057] Silica 5.0g
[0058] Magnesium Stearate 3.5g
[0059] Coating prescription:
[0060] Gastric film coating premix 7.8g
[0061] Purified water 70.2ml
[0062] Preparation Process:
[0063] (1) Pass the minocycline hydrochloride through a 100-mesh sieve, pass the auxiliary materials through a 80-mesh sieve, and set aside.
[0064] (2) Weigh the prescribed amount of minocycline hydrochloride, hypromellose (E50LV), and lactose, mix well, and dry-press the granules with a dry granulator (extrusion speed: 20r / min; pressure: 3.0MPa) , Sieve the 16-30 mesh particles, and add the sifted fine powder to the granulation cycle.
[0065] (3) Add the prescribed amount of silicon dioxide and magnesium stearate, mix well, and press plain tablets.
[0...
Embodiment 2
[0068] Embodiment 2: a kind of minocycline hydrochloride sustained-release tablet, its prescription is as follows:
[0069] Minocycline hydrochloride 45.0g (as minocycline)
[0070] Hypromellose (E50LV) 95.0g
[0071] Mannitol 230.0g
[0072] Silica 5.0g
[0073] Magnesium Stearate 3.5g
[0074] Coating prescription:
[0075] Gastric film coating premix 7.8g
[0076] Purified water 70.2ml
[0077] Preparation Process:
[0078] (1) Pass the minocycline hydrochloride through a 100-mesh sieve, pass the auxiliary materials through a 80-mesh sieve, and set aside.
[0079] (2) Weigh the prescribed amount of minocycline hydrochloride, hypromellose (E50LV), and mannitol, mix well, and dry-press the granules with a dry granulator (extrusion speed: 20r / min; pressure: 3.0MPa ), sieve 16-30 mesh granules, and the sieved fine powder is added to the granulation cycle.
[0080] (3) Add the prescribed amount of silicon dioxide and magnesium stearate, mix well, and press plain tablets...
Embodiment 3
[0083] Embodiment 3: a kind of minocycline hydrochloride slow-release tablet, its prescription is as follows:
[0084] Minocycline hydrochloride 45.0g (as minocycline)
[0085] Hypromellose (E50LV) 95.0g
[0086] Lactose 230.0g
[0087] Silica 5.0g
[0088] Magnesium Stearate 3.5g
[0089] Coating prescription:
[0090] Gastric film coating premix 7.8g
[0091] Purified water 70.2ml
[0092] Preparation Process:
[0093] (1) Pass the minocycline hydrochloride through a 100-mesh sieve, pass the auxiliary materials through a 80-mesh sieve, and set aside.
[0094] (2) Weigh the prescribed amount of minocycline hydrochloride, hypromellose (E50LV), and lactose, mix well, and dry-press the granules with a dry granulator (extrusion speed: 20r / min; pressure: 2.5MPa) , Sieve the 16-30 mesh particles, and add the sifted fine powder to the granulation cycle.
[0095] (3) Add the prescribed amount of silicon dioxide and magnesium stearate, mix well, and press plain tablets.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com