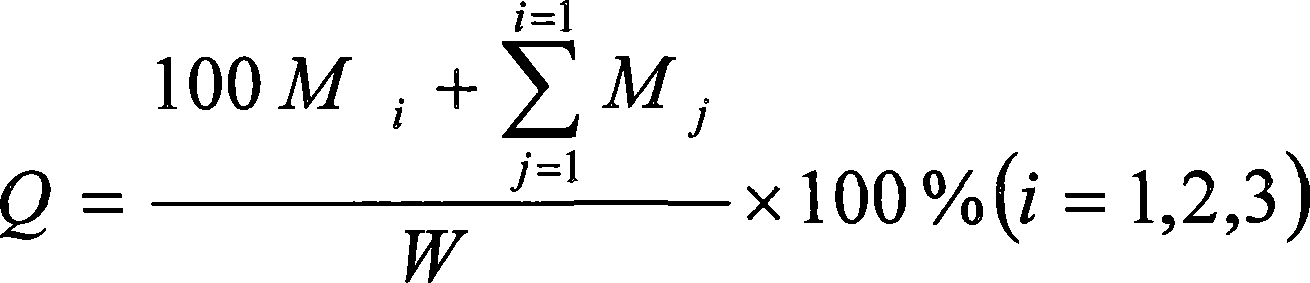Controlled ointment compound stroma and the preparing method
A technology of ointment and matrix, which is applied in the field of medicine and can solve problems such as cumbersome production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Grind 5g of chlorhexidine acetate and 15g of glycerin in a mortar for 5 minutes. After grinding evenly, add 30g of β-cyclodextrin and grind them together for 15 minutes to obtain clathrates. At this time, 5g of glycerol triacetate and 26g Diethyl phthalate plasticizer was mixed and heated at 150°C for 15 minutes to melt the mixture of 14g ethyl cellulose and 5g RSPO into the clathrate and grind together for 30 minutes to obtain a controlled release ointment base.
Embodiment 2
[0035] Grind 5g of metronidazole and 5g of glycerin in a mortar for 5 minutes, and after grinding evenly, add 5g of β-cyclodextrin into it and grind for 15 minutes to obtain an inclusion compound. At this time, 5g of triacetin and 40g of Diethyl phthalate plasticizer was mixed and heated at 150° C. for 15 minutes to melt 40 g of ethyl cellulose, added to the clathrate and co-ground for 90 minutes to prepare a controlled-release ointment base.
Embodiment 3
[0037] Grind 5g of metronidazole and 15g of glycerin in a mortar for 5 minutes, and after grinding evenly, add 25g of 2,3-diacetyl-β-cyclodextrin into it and grind together for 15 minutes to obtain an inclusion compound. 7g glyceryl triacetate was mixed with 30g dibutyl phthalate plasticizer and heated at 150°C for 15 minutes to melt, and 18g ethyl cellulose was added to the clathrate and ground together for 60 minutes to obtain a controlled release ointment matrix.
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com