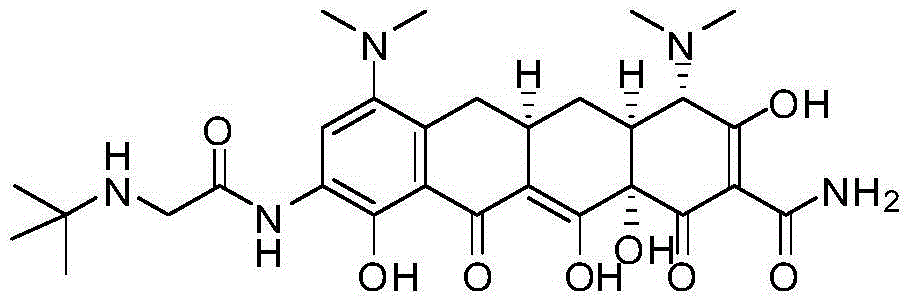
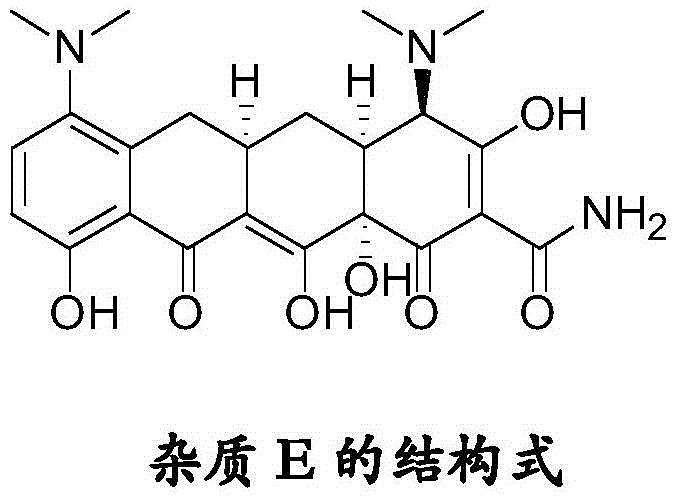
Tigecycline impurity stereoselective preparation method
A technology of stereoselectivity and tigecycline, which is applied in the field of preparation of tigecycline impurity E, can solve problems such as no literature reports in the preparation, and achieve the effects of good stereoselectivity, improved quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 500 ml of methanol and 40 ml of acetic acid into a 1 L reaction bottle, and add 40 g of minocycline hydrochloride (source: North China Pharmaceutical Co., Ltd., the same below) under stirring. Raise the temperature to 40°C, hold the reaction for 3 hours, stop heating, lower the temperature to -5-5°C, control the temperature and add triethylamine dropwise, adjust the pH value to 7.5-8.0, precipitate a light yellow solid, control the temperature at 0-5°C and stir for 2 hours, The pH value did not change again, filtered, washed the filter cake with methanol, and dried under reduced pressure at room temperature for 4 hours to obtain 19.4 g of tigecycline impurity E with a purity of 98.2% and a yield of 52.5%.
[0023] The condition and the method (the same below) that HPLC method detects tigecycline impurity E purity are:
[0024] Chromatographic column: ODS-BP C18 (4.6×250mm 5μm);
[0025] Mobile phase: acetonitrile—0.05mol / L ammonium dihydrogen phosphate solution (ad...
Embodiment 2
[0036] Add 500ml of methanol and 38ml of formic acid into a 1L reaction flask, and add 40g of minocycline hydrochloride under stirring. Raise the temperature to 40°C, keep it warm for 3 hours, stop heating, lower the temperature to -5-5°C, control the temperature and add methylamine dropwise, adjust the pH value to 7.5-8.0, precipitate a light yellow solid, control the temperature at 0-5°C and stir for 2 hours, repeat There was no change in the pH value, filtered, washed the filter cake with methanol, and dried under reduced pressure at room temperature for 4 hours to obtain 20 g of tigecycline impurity E with a purity of 98.5% and a yield of 54.2%.
Embodiment 3
[0038] Add 500ml of ethanol and 40ml of formic acid into a 1L reaction flask, and add 40g of minocycline hydrochloride under stirring. Raise the temperature to 40°C, hold the reaction for 3 hours, stop heating, lower the temperature to -5-5°C, control the temperature and add sodium carbonate, adjust the pH value to 7.6-8.0, precipitate a light yellow solid, control the temperature at 0-5°C and stir for 2 hours, retest The pH value remained unchanged, filtered, washed the filter cake with ethanol, and dried under reduced pressure at room temperature for 4 hours to obtain 19.6 g of tigecycline impurity E with a purity of 98.2% and a yield of 53%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com