Tigecycline purification method
A technology of tigecycline and purification method, which is applied in the field of medicinal chemistry, can solve problems such as not being able to meet quality standards, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
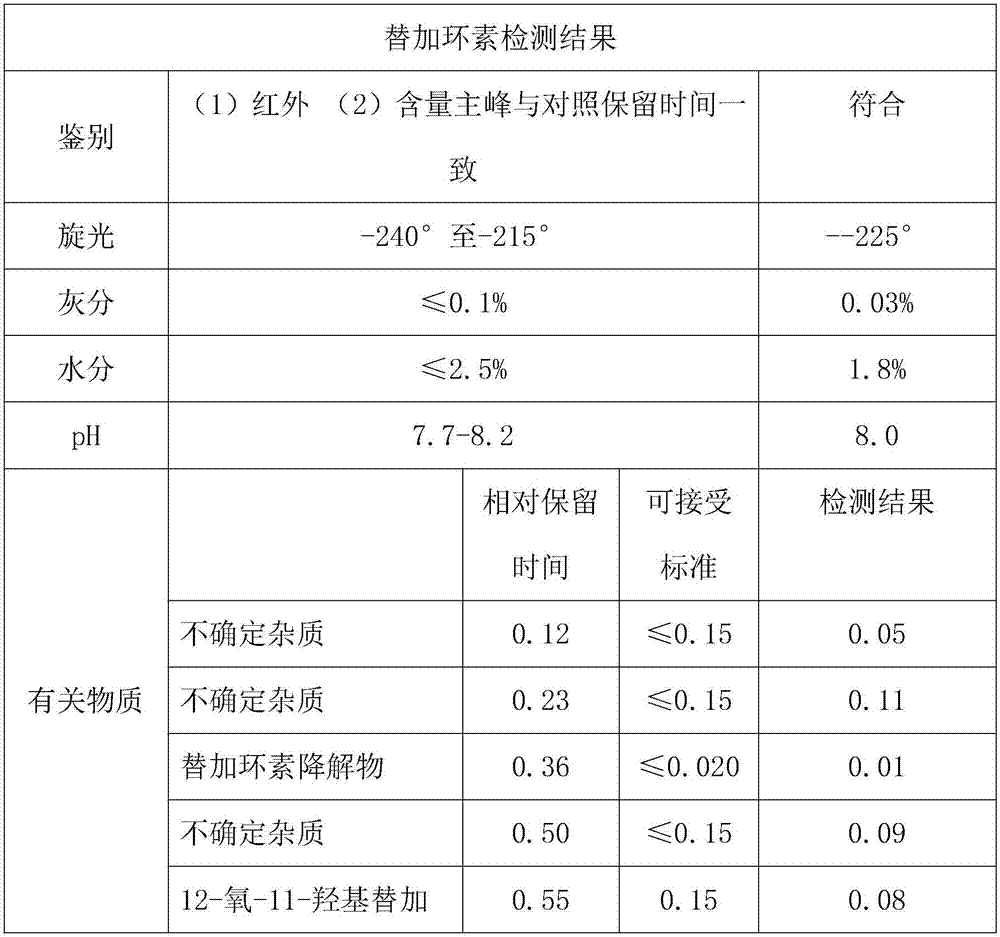
Embodiment 1
[0020] Add 10g, 0.03mol of aminominocycline hydrochloride to 0-5°C, 75ml of deionized water, and add 16g, 0.1mol of tert-butylaminoglycyl chloride hydrochloride in portions under nitrogen protection, and stir to react 1h, after the reaction is complete, add 14% ammonia water to adjust the pH to 7.2, add methanol 140ml, and extract with an organic solvent of a mixture of ether, ethyl acetate, n-heptane and petroleum ether, and then add 3% ammonia water to adjust the pH to 7.2 , after stirring for 15 minutes, the liquids were separated, the aqueous phase was extracted with a mixed aqueous solution of methanol, ethanol and acetone, the organic phases were combined, washed 2 to 4 times with 40-50% methanol, dried over anhydrous sodium sulfate and filtered, and the filter cake was washed with two Wash with methyl chloride, combine the filtrate and lotion, concentrate under reduced pressure at 25-35°C, and obtain crude tigecycline as a brown solid after vacuum drying. Add the crude t...
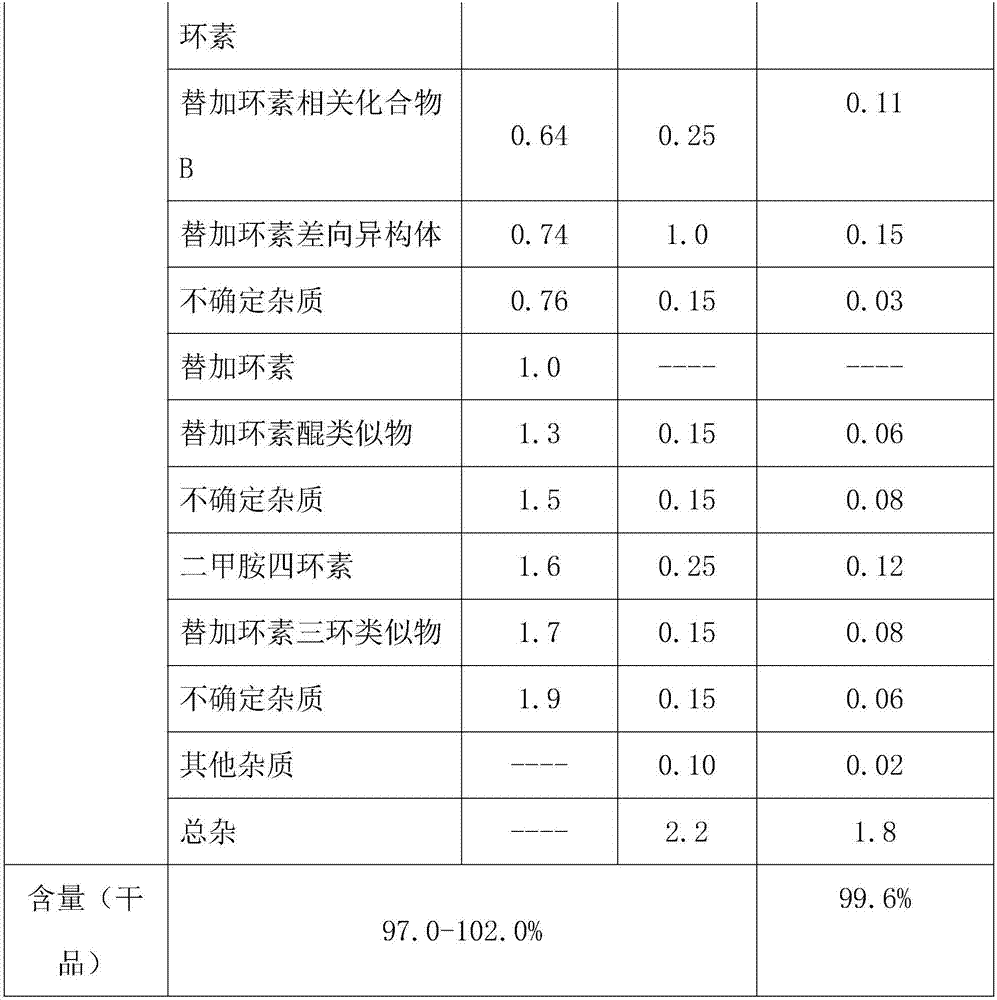
Embodiment 2
[0024] Add 14g, 0.03mol of aminominocycline hydrochloride to 0-5°C, 75ml of deionized water, and add 20g, 0.1mol of tert-butylaminoglycyl chloride hydrochloride in portions under nitrogen protection, and stir to react 1.2h, after the reaction is complete, add 20% ammonia water to adjust the pH to 7.2, add methanol 200ml, and extract with an organic solvent of a mixture of ether, ethyl acetate, n-heptane and petroleum ether, then add 9% ammonia water to adjust the pH to 7.2, after stirring for 20 minutes, separate the liquids, extract the aqueous phase with a mixed aqueous solution of methanol, ethanol and acetone, combine the organic phases, wash with 50% methanol for 3 times, dry with anhydrous sodium sulfate overnight and filter, and wash the filter cake with dichloromethane , combined the filtrate and lotion, concentrated under reduced pressure at 28°C, and obtained brown solid tigecycline crude product after vacuum drying, added the crude tigecycline product to 100ml of eth...
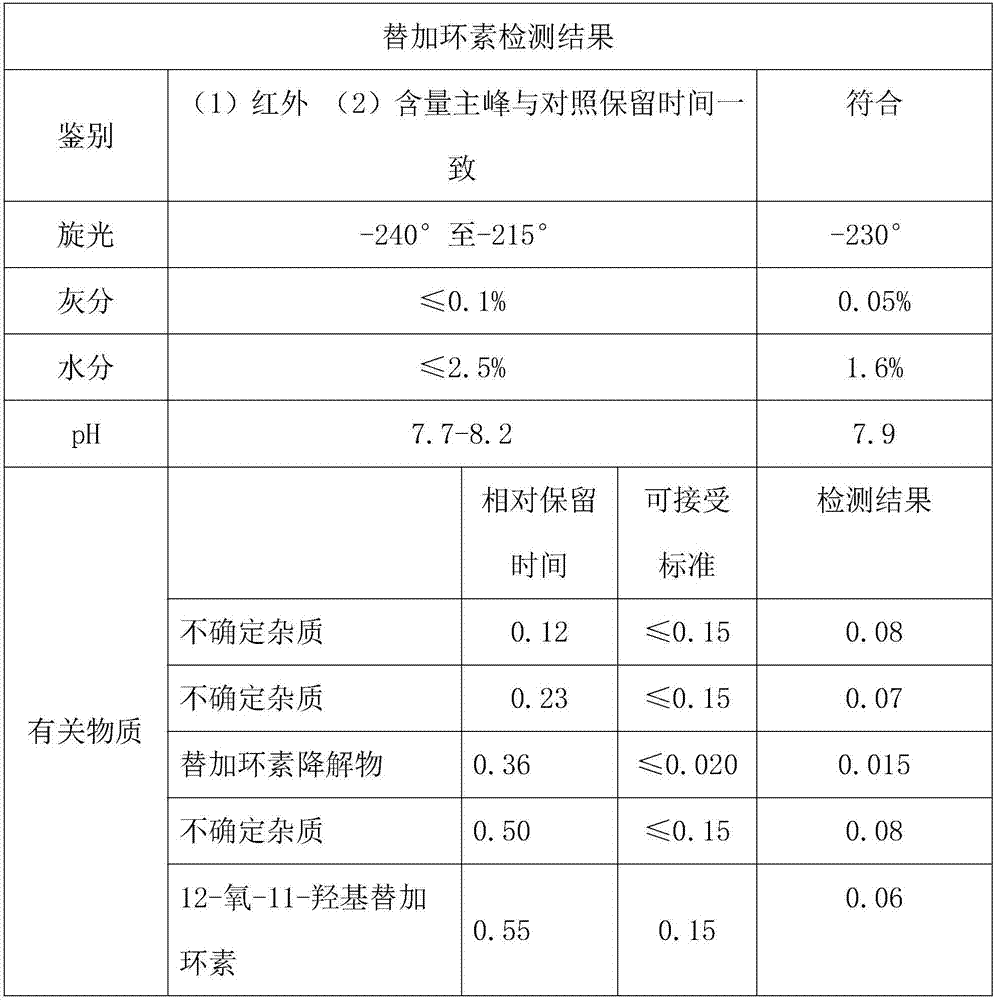
Embodiment 3
[0027] Example 3 Aminominocycline hydrochloride 14g, 0.03mol was added to 0-5°C, 75ml of deionized water, and tert-butylaminoglycyl chloride hydrochloride 18g, 0.1mol was added in portions under nitrogen protection , stirred and reacted for 1.5h, after the reaction was complete, add 18% ammonia water to adjust the pH to 7.2, add methanol 140ml, and extract with an organic solvent of a mixture of ether, ethyl acetate, n-heptane and petroleum ether, and then add 6% ammonia water Adjust the pH to 7.2, stir for 18 minutes, then separate the liquids, extract the aqueous phase with a mixed aqueous solution of methanol, ethanol and acetone, combine the organic phases, wash 4 times with 45% methanol, dry overnight with anhydrous sodium sulfate, and filter the filter cake with two Wash with methyl chloride, combine the filtrate and lotion, concentrate under reduced pressure at 30°C, and obtain the crude product of tigecycline as a brown solid after vacuum drying. Add the crude product o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com