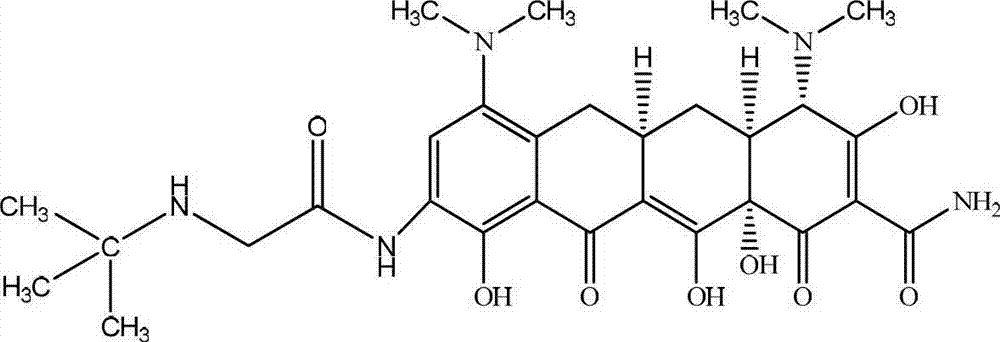
Preparation method of high-purity tigecycline
A tigecycline, high-purity technology, applied in the field of preparation of high-purity tigecycline, can solve the problems of large amount of solvent used, unstable intermediates, epimerization, etc., and achieve reasonable hydrogenation pressure, Effects of shorter purification process time and reduced solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
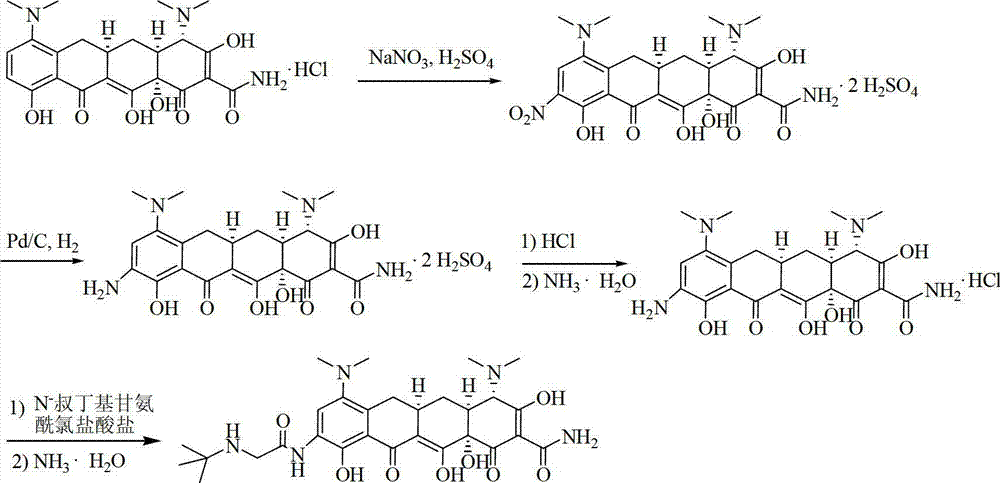
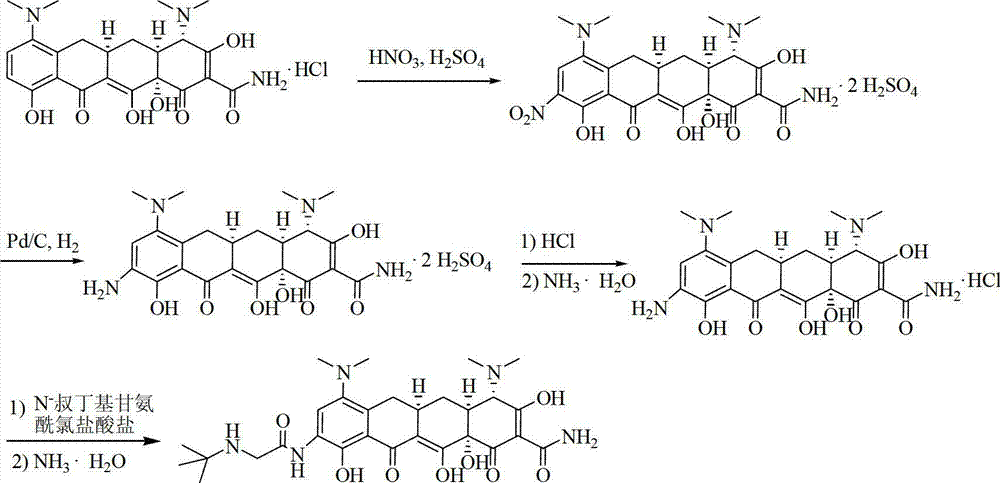
[0043] Under nitrogen protection, at 0°C, dissolve 10.0 g of minocycline hydrochloride in 28 ml (51.52 g) of concentrated sulfuric acid; stir to exhaust the HCl in the system; slowly add 1.9 g of fuming nitric acid dropwise, and stir for 5 hours; after the reaction , the reaction solution was slowly added to isopropanol / n-heptane (mass ratio 7:1) mixed solution 250g, stirred at room temperature for 1 hour; filter, filter cake with isopropanol / n-heptane (mass ratio 7:1) The mixture was washed (16 g x 3 times) and drained; the filter cake was dried under reduced pressure at 40°C for 5 hours to obtain 12.66 g of yellow powder with a yield of 89.5%.
Embodiment 2
[0045] Under nitrogen protection, at 0°C, dissolve 4.85kg of minocycline hydrochloride in 15L of concentrated sulfuric acid; stir to exhaust the HCl in the system; slowly add 1.07kg of fuming nitric acid dropwise, and stir at 0°C for 4.5-5.5 hours; after the reaction , slowly add the reaction solution to isopropanol / n-heptane (volume ratio 7:1) mixed solution 160L, stir at room temperature for 1-1.5 hours; filter, filter cake with isopropanol / n-heptane (volume ratio 7:1 1) The mixture was washed (10 L x 3 times), and drained; the filter cake was dried under reduced pressure at 40°C for 5 hours to obtain 6.24 kg of yellow powder, with a yield of 90.9%.
Embodiment 3
[0047] Basically the same as Example 2, the difference is that the dispersant and crystallizer are isopropanol / n-heptane / cyclohexane (volume ratio 7:1:4), and finally 6.49kg of yellow powder is obtained, with a yield of 94.5% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com