Guanidine hydrochloride sustained release preparation and preparation method thereof
A technology of guanfacine hydrochloride and sustained-release preparations, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The pharmacokinetic parameters such as the degree of availability are not ideal, so as to achieve the effect of less toxic and side effects, high production efficiency, and convenient long-term treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The raw and auxiliary materials of the guanfacine hydrochloride sustained-release preparation of the present invention consist of:
[0049] Taking g as the unit, the weight of each raw and auxiliary material in the original formula of guanfacine hydrochloride sustained-release preparation was enlarged by 5 to 15 times. 232.5g of lactose, 75g of fumaric acid, 250g of hydroxypropyl methylcellulose, 175g of polyacrylic resin, 3g of micronized silica gel, 3g of magnesium stearate and 120g of water.
[0050] The preparation method of guanfacine hydrochloride slow-release preparation of the present invention:
[0051] Prepare various raw materials and auxiliary materials according to the composition of raw materials and auxiliary materials of the above-mentioned guanfacine hydrochloride sustained-release tablets, prepare guanfacine hydrochloride 11.48g, lactose 232.5g, fumaric acid 75g, hydroxypropyl methylcellulose 250g, polyacrylic acid 175g of resin was crushed until it p...
Embodiment 2
[0052] Embodiment 2: basically the same as Embodiment 1, the difference is:
[0053] The raw and auxiliary materials of the guanfacine hydrochloride sustained-release preparation of the present invention consist of:
[0054] Taking g as the unit, the weight of each raw and auxiliary material in the original formula of the guanfacine hydrochloride sustained-release preparation was enlarged by 5 to 15 times. Mannitol 190g, citric acid 100g, hydroxypropyl cellulose 300g, polyacrylic resin 135g, micropowder silica gel 3g, sodium stearyl fumarate 5g and 5% povidone aqueous solution 100g.
Embodiment 3
[0055] Embodiment 3: basically the same as Embodiment 1, the difference is:
[0056] Taking g as the unit, the weight of each raw and auxiliary material in the original formula of guanfacine hydrochloride sustained-release preparation was enlarged by 5 to 15 times. Lactose 200g, microcrystalline cellulose 30g, fumaric acid 100g, ethyl cellulose 250g, polyacrylic resin 150g, micropowder silica gel 3g, magnesium stearate 3g and water 120g. .
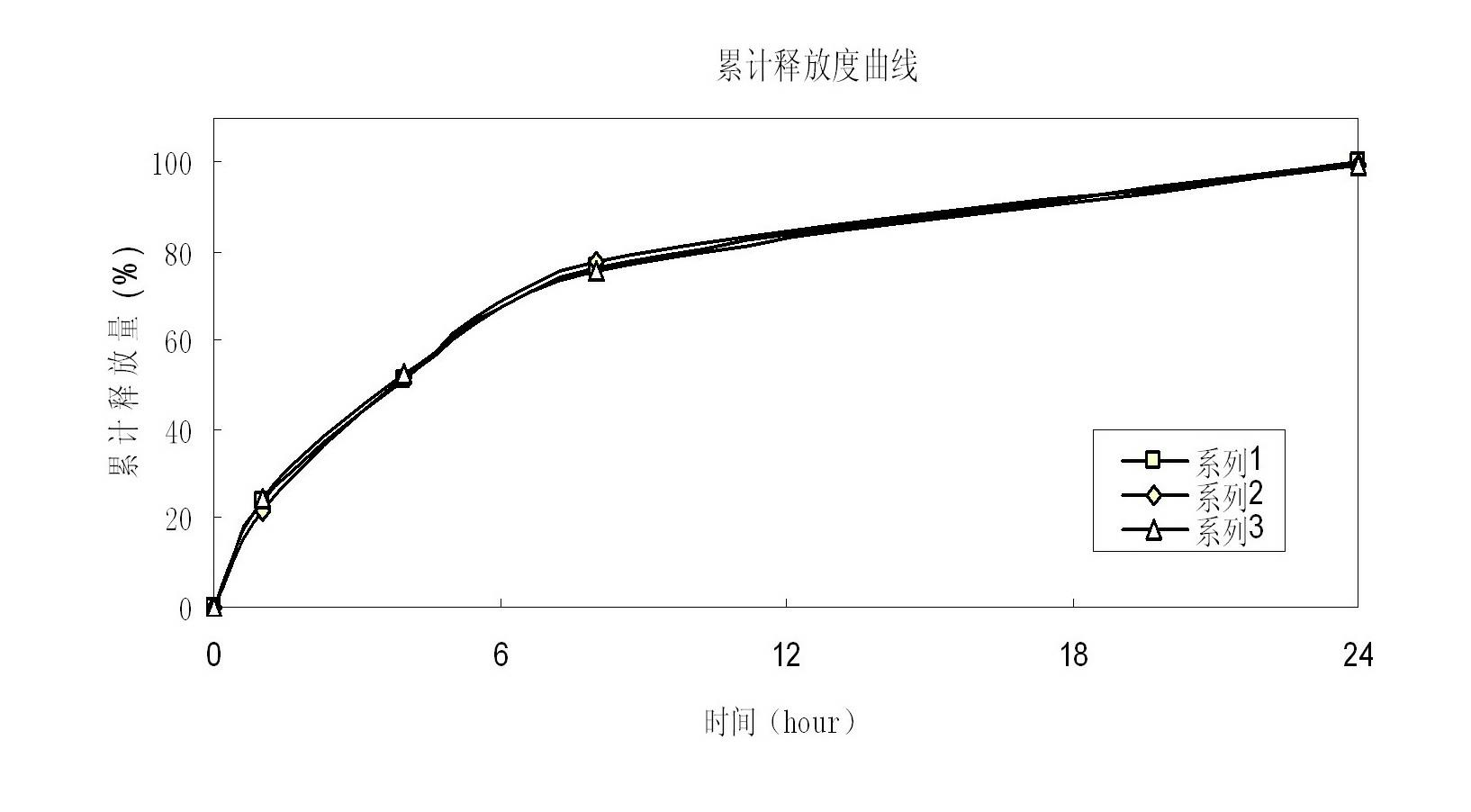
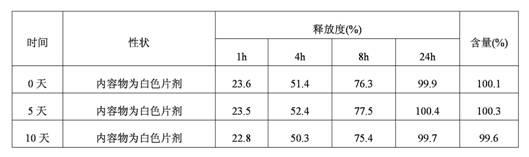
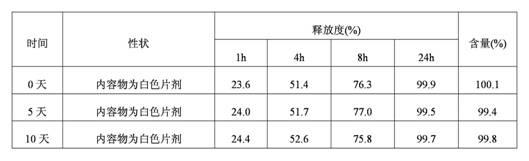
[0057] Taking the guanfacine hydrochloride sustained-release tablet prepared in Example 1 as the test sample, the release test method is as follows:
[0058] Get the product obtained in Example 1, according to the release assay method (the second method of two appendix X D of Chinese Pharmacopoeia 2010 edition), adopt the dissolution assay device, the rotating speed is 100rpm, and temperature is 37 ℃, and release medium is the hydrochloric acid buffering of pH 2.2 Liquid 900ml, the sampling time points are: 1 hour, 4 hours, 8 hours and 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com