Lentiviral vector suitable for gene therapy of thalassemia and sickle anemia
A technology of lentiviral vector and gene locus, which is applied in the medical field, can solve the problem of virus titer reduction and other problems, and achieve the effect of preventing gene activation and maintaining gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
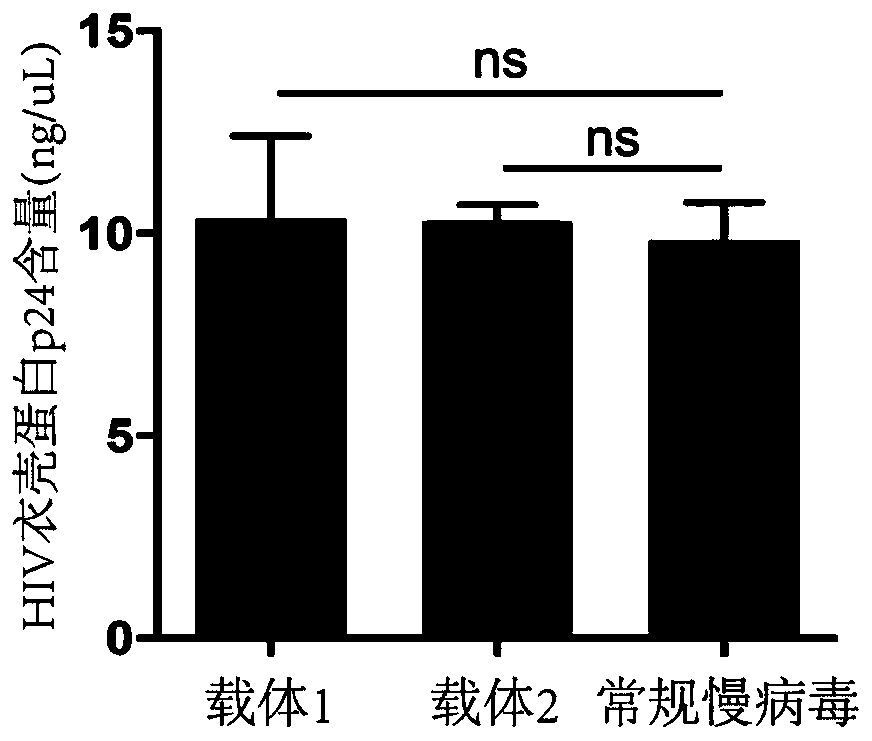
Examples
Embodiment 1
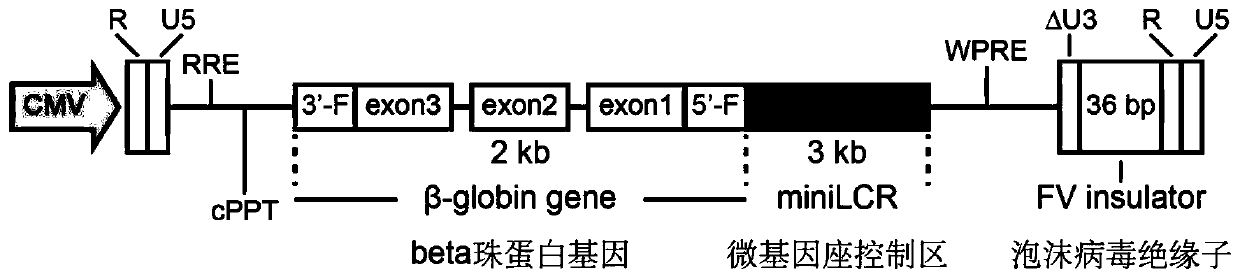
[0038] The present invention screens an expression box of human beta globin with red blood cell specificity and high-efficiency expression, and then obtains a lentiviral vector containing the expression box for gene therapy. Carrier design such as figure 1 As shown, the expression cassette comprises the following elements: (1) mini-locus control region (miniLCR), that is, a length 3kb (sequence such as shown in SEQ ID NO.1) or 2.6kb (sequence shown in SEQ ID NO.2) regulatory element; (2) gene sequence of beta globin (sequence shown in SEQ ID NO.3); Son 2 contained the T87Q mutation. (3) 265-bp beta globin gene sequence upstream promoter sequence (sequence shown in SEQ ID NO.4); (4) downstream 300-bp beta globin gene sequence polyA sequence (sequence as SEQ ID shown in NO.5). Place the expression cassette in a lentiviral vector. The sequence of the lentiviral vector containing the 3kb minilocus control region is shown in SEQ ID NO.7. The sequence of the lentiviral vector c...
Embodiment 2
[0041] The present invention screens an expression box of human beta globin with red blood cell specificity and high-efficiency expression, and then obtains a lentiviral vector containing the expression box for gene therapy. Carrier design such as figure 1 As shown, the expression cassette comprises the following elements: (1) mini-locus control region (miniLCR), that is, a length 3kb (sequence such as SEQ ID NO.1) or a 2.6kb (sequence shown in SEQ ID NO.2) regulatory element; (2) the complete beta globin gene sequence. Among them, exon 2 contains T87Q mutation. (3) 265-bp beta globin gene sequence upstream promoter sequence (sequence shown in SEQ ID NO.4); (4) downstream 300-bp beta globin gene sequence polyA sequence (sequence as SEQ ID shown in NO.5). This expression cassette is placed in a lentiviral vector, which selectively adds the insulator sequence. In the case of adding an insulator sequence, a 36bp insulator sequence from Foamy virus (sequence shown in SEQ ID NO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com