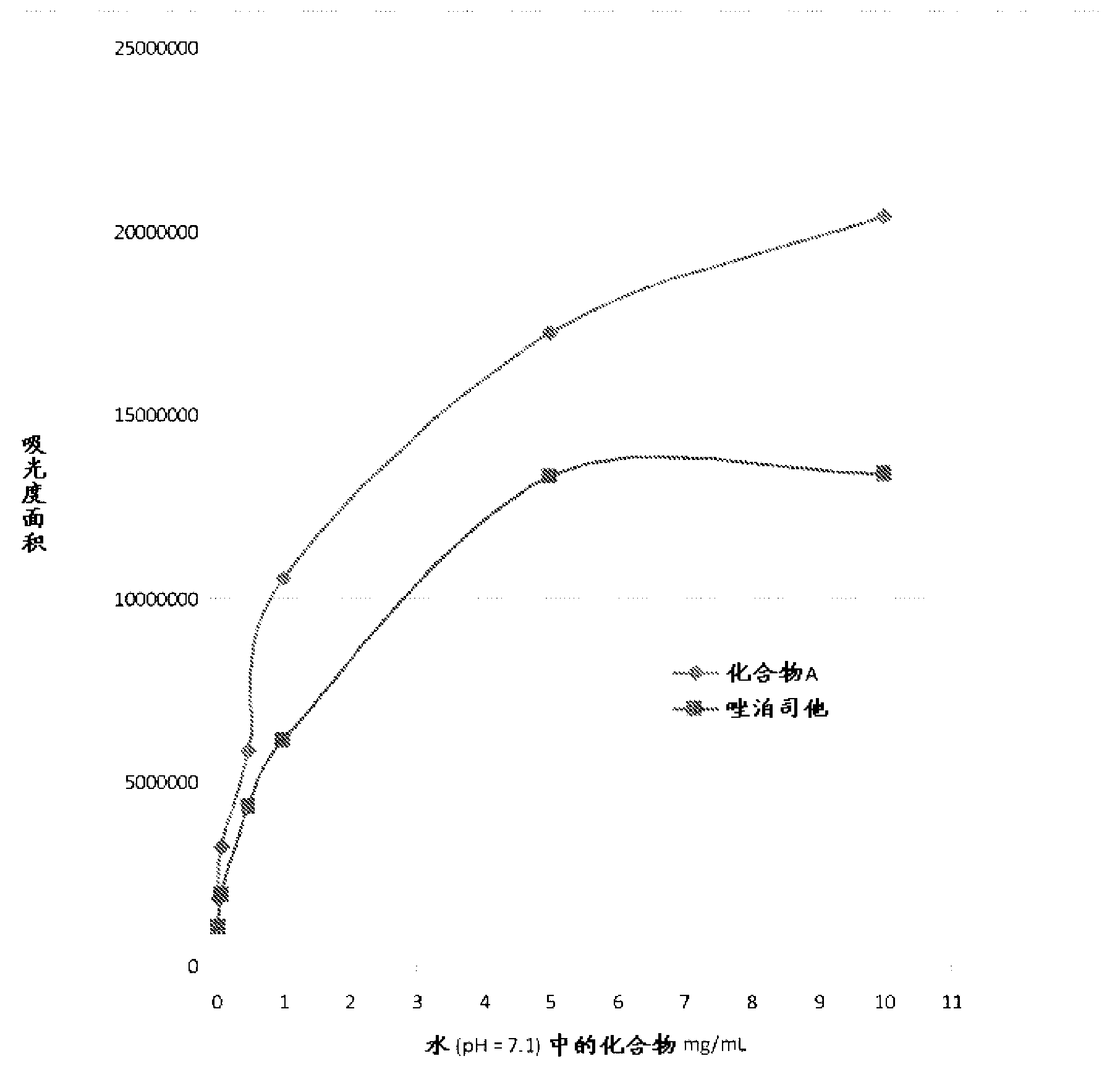
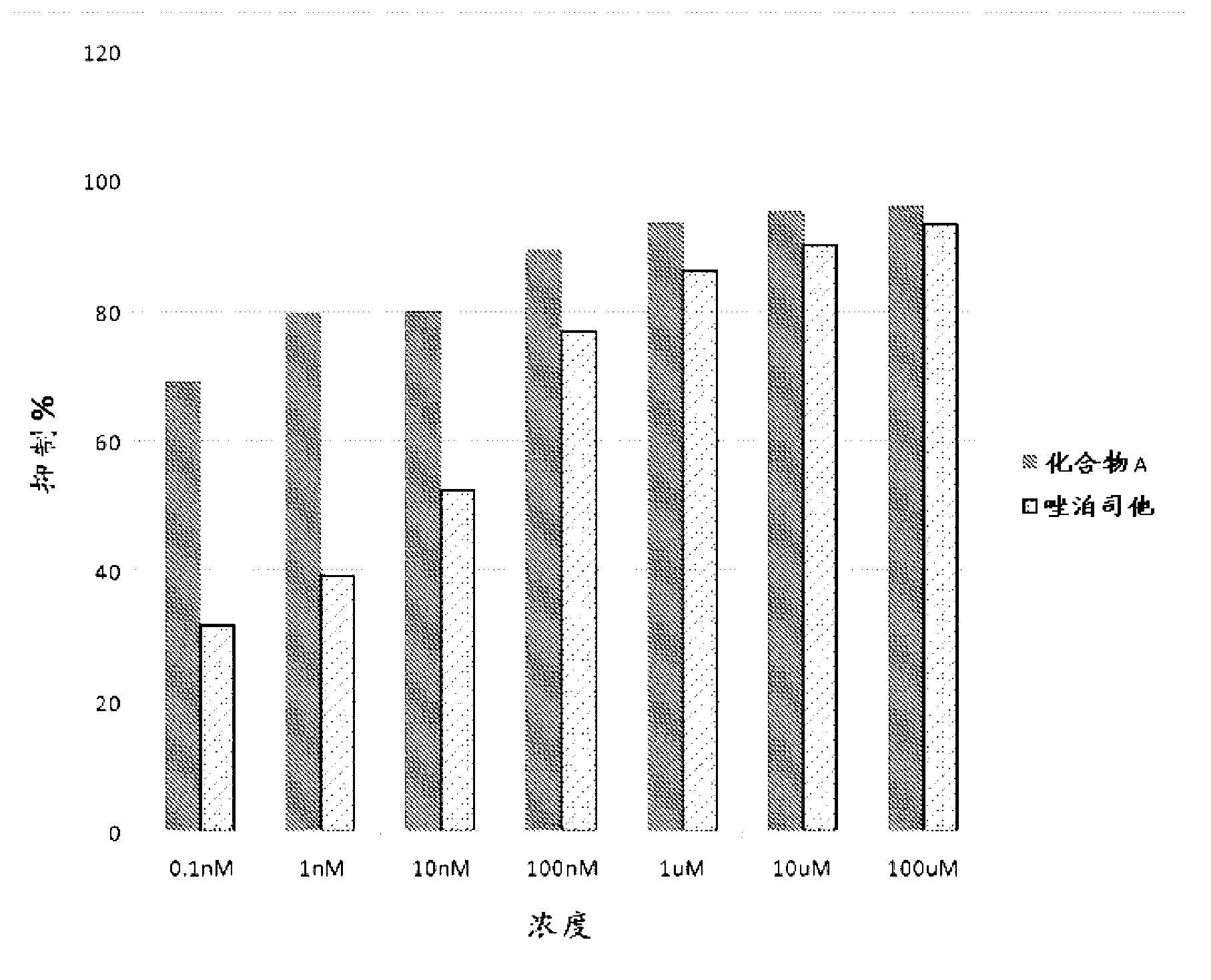
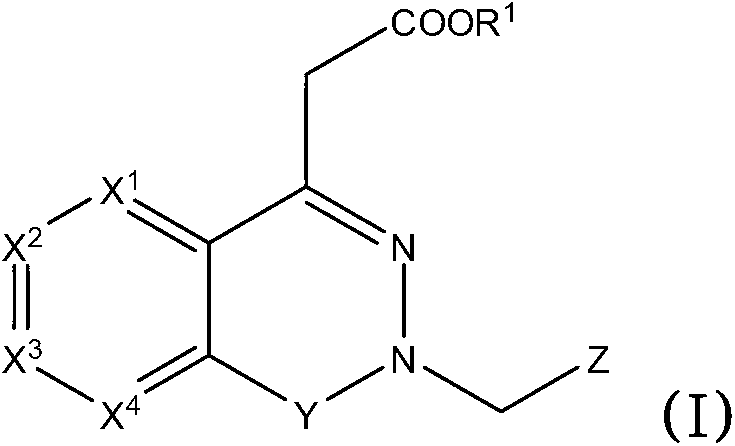
Aldose reductase inhibitors and uses thereof
A technology of alkyl and compound, applied in the field of aldose reductase inhibitors and its application, can solve the problems of excess sorbitol and difficult diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0252] Example 1: Preparation of Compound A.
[0253] Compound A was prepared as illustrated schematically below.
[0254]
[0255] Preparation of (E) / (Z)-2-(7-oxofuro[3,4-b]pyrazine-5(7H)-ylidene) tert-butyl acetate (compound 1)
[0256] To stirred 5.05 g (33.63 mmol) of commercially available 2,3-pyrazine dicarboxylic anhydride in 300 mL CHCl 3 12.34 g (33.63 mmol) (tert-butoxycarbonylmethylene)-triphenylphosphorane were added to the solution. The resulting solution was heated to 62 °C for 2 days. The reaction mixture was concentrated in vacuo, and the residue was purified by flash column chromatography on silica gel (monitored by thin layer chromatography) and eluted with 1:1 (v / v) hexane:ethyl acetate. Evaporation of the collected fractions yielded 2.99 g (36% yield) of (E) / (Z)-2-(7-oxofurano as a mixture (~1:1) of unresolved geometric isomers [3,4-b]pyrazine-5(7H)-ylidene) tert-butyl acetate (compound 1): 1 HNMR (CDCl 3 ,300MHz):δ ppm 9.03(d,J=2.4Hz,1H),8.96(d,J...
Embodiment 2
[0263] Example 2: Alternative Preparation of Compound A.
[0264]
[0265] Preparation of 2-(iodomethyl)-5-(trifluoromethyl)benzo[d]thiazole (Compound 5)
[0266] To a stirred solution of 10.79 g (42.97 mmol) of 2-(chloromethyl)-5-(trifluoromethyl)benzo[d]thiazole (compound 3) in 86 mL of acetone was added 7.40 g (49.42 mmol) of sodium iodide . The resulting reaction mixture was heated to 55 °C for 1 hour. The reaction mixture was cooled to ambient temperature and concentrated under vacuum. The residue was partitioned between EtOAc and water, the layers were separated, and the organic layer was washed with water (1x). The recovered organic layer was then washed with 1.0M Na 2 S 2 o 3 Process and stir vigorously for 15 minutes. The layers were then separated and the organic layer was washed sequentially with water (1x) and brine (1x). The organic layer was washed with Na 2 SO 4 Dried, filtered and concentrated under vacuum to yield 14.28 g (97% crude yield) of 2-(i...
Embodiment 3
[0278] Example 3: Preparation of Compound 8.
[0279] Compound 8 shown below was prepared as follows:
[0280]
[0281] Except that 5-chloro-2-(chloromethyl)-benzo[d]thiazole was the reagent used instead of 2-(chloromethyl)-5-(trifluoromethyl)benzo[d]thiazole, The preparation described for compound 4 was repeated using the same molar ratios as previously described. In this case, the final product obtained was 2-(7-((5-chlorobenzo[d]thiazol-2-yl)methyl)-8-oxo-7,8-di Hydropyrazino[2,3-d]pyridazin-5-yl) tert-butyl acetate (compound 8): 1 HNMR (CDCl 3 ,300MHz): δ ppm 9.08(s,1H),9.05(s,1H),8.00(s,1H),7.74(d,J=8.7Hz,1H),7.36(d,J=8.7Hz,1H),5.87(s,2H ), 4.04(s,2H), 1.41(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com