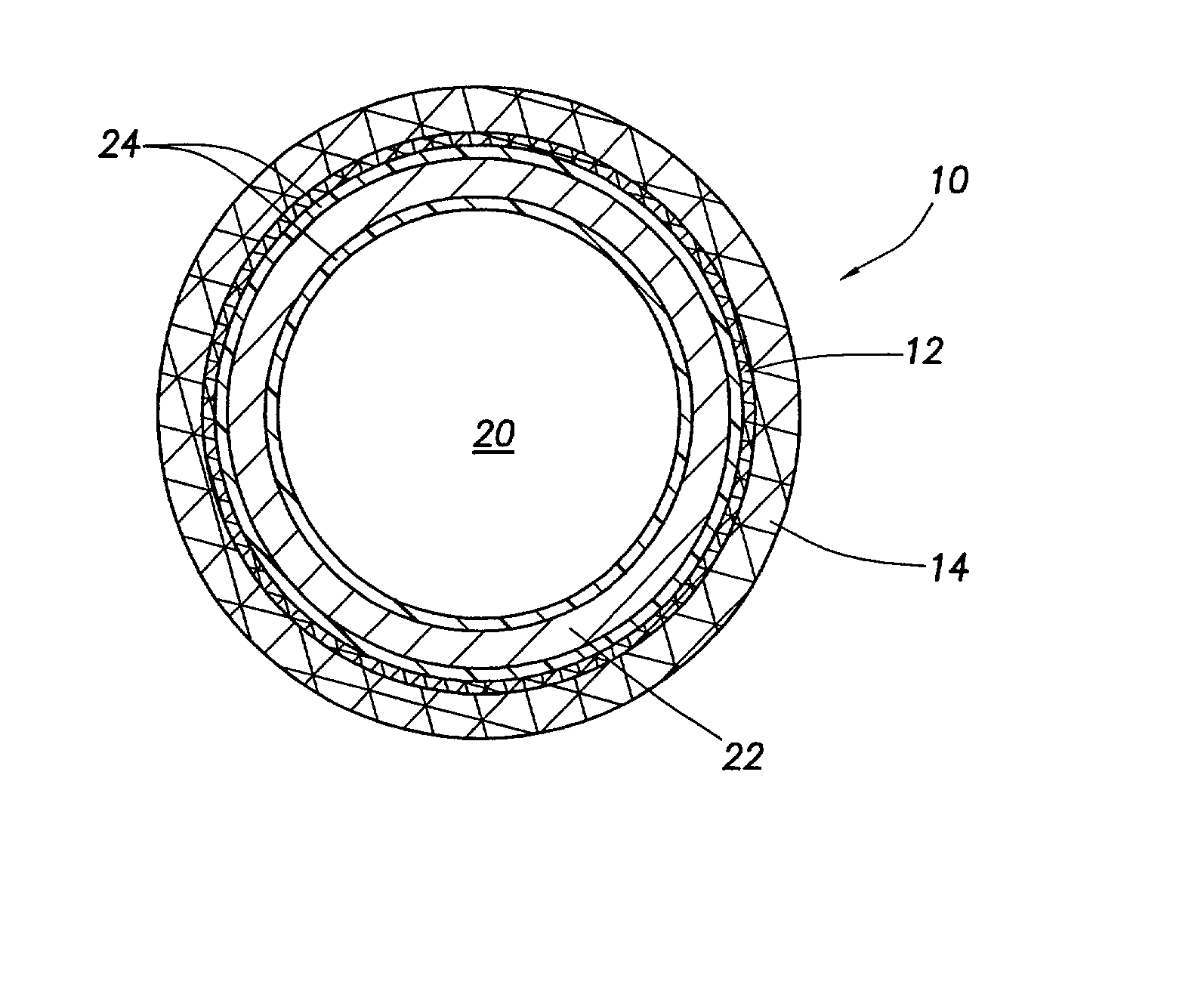
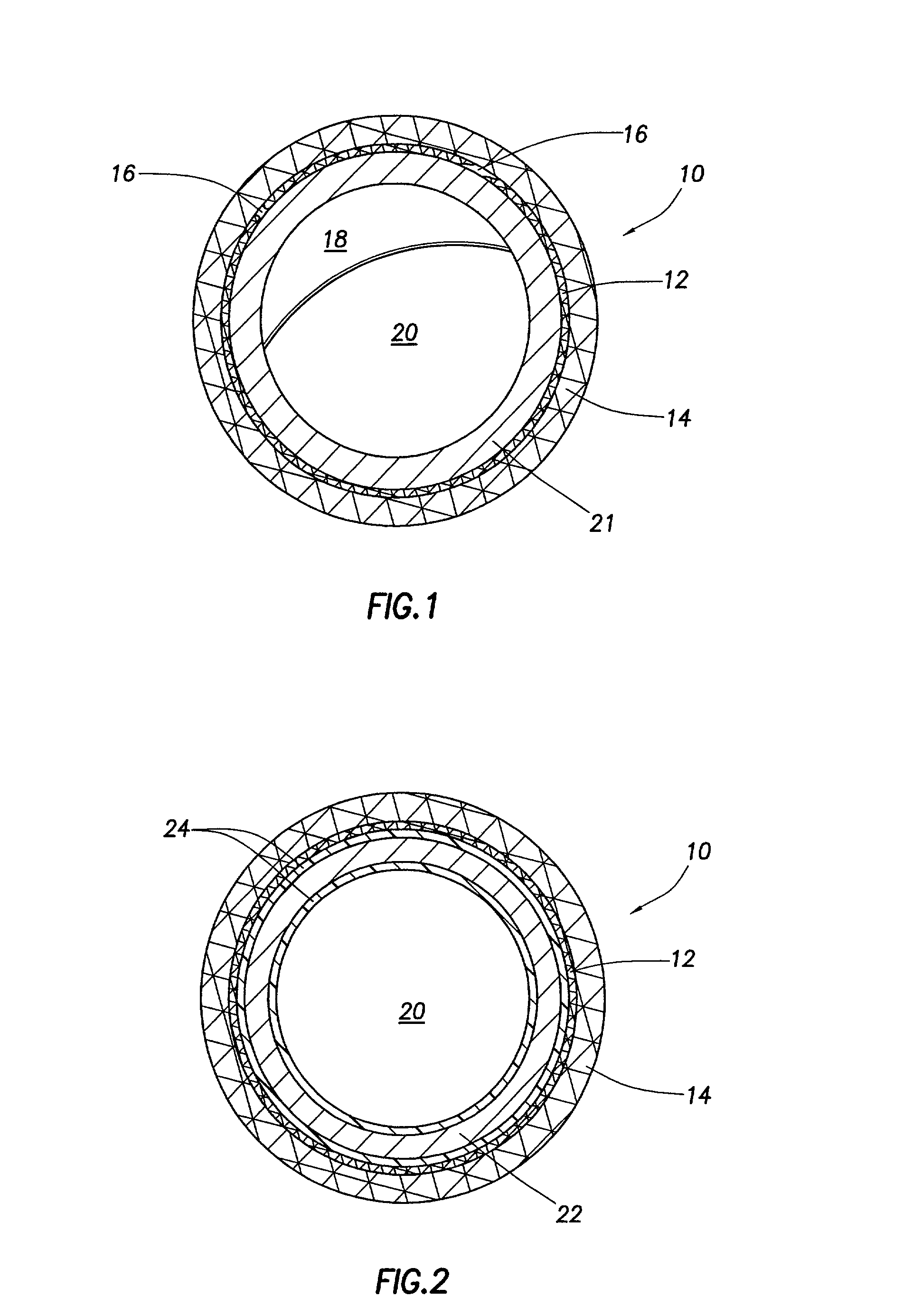
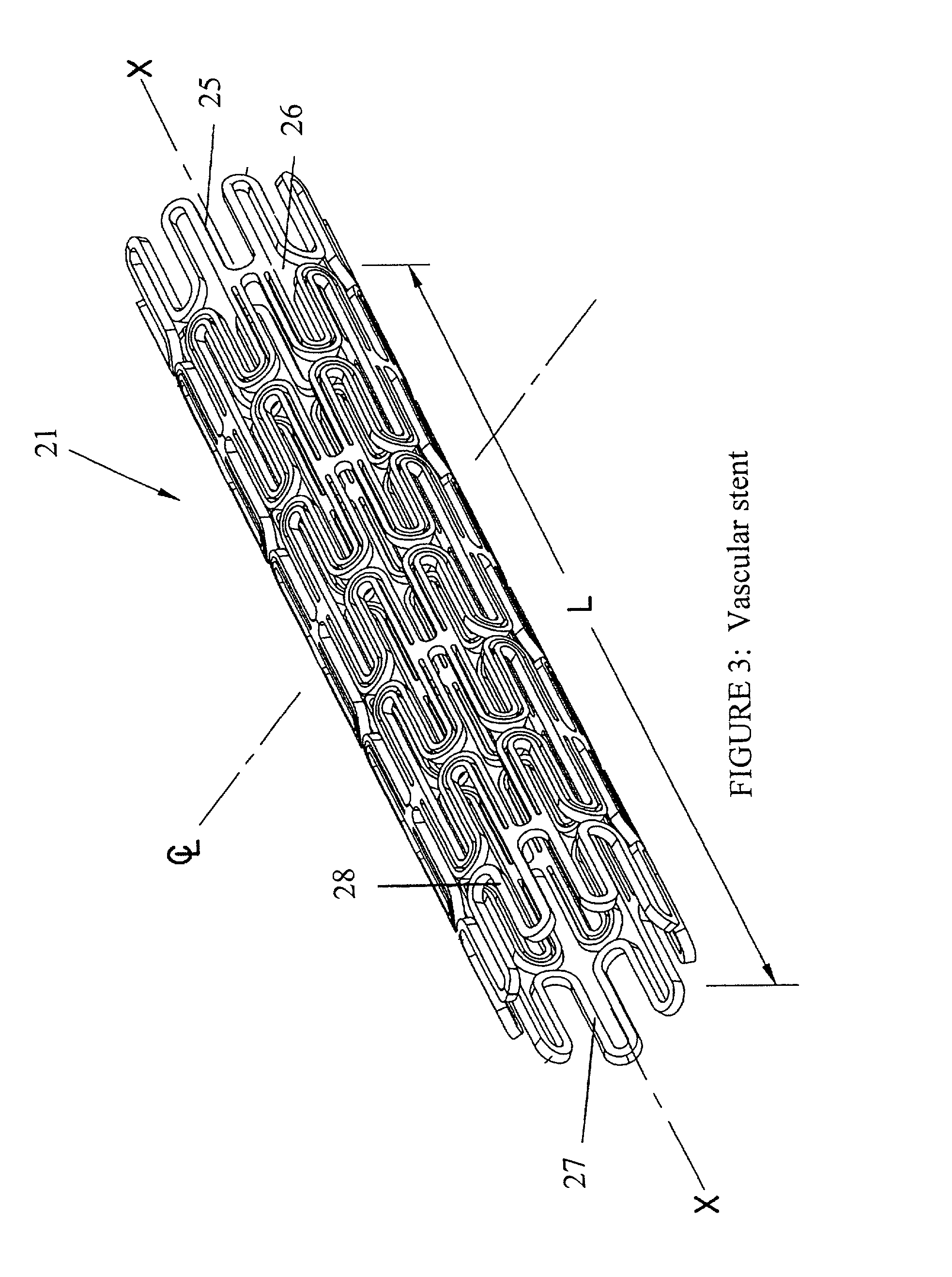
Stent coatings containing HMG-CoA reductase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0057] One hundred (100) mg PCL (poly caprolactone) polymer and 10 mg of cerivastatin were dissolved in 10 ml methylene chloride solution at room temperature. The solution was poured onto a glass plate and the solvent was allowed to evaporate for 12-24 hours. After almost complete removal of the solvent, the cerivastatin-loaded PCL film was removed from the glass plate and was cut to 1.5 cm by 1.5 cm size. The film was mounted on a Palmaz-Schatz coronary endovascular stent. Control PCL films were prepared in the following manner: 100 mg PCL (poly caprolactone) polymer was dissolved in 10 ml methylene chloride solution at room temperature. The solution was poured onto a glass plate and the solvent was allowed to evaporate for 12-24 hours. After almost complete removal of the solvent, the control PCL film was removed from the glass plate and was cut to 1.5 cm by 1.5 cm size. The control film was mounted on a Palmaz-Schatz coronary endovascular stent. Release profiles were obtained fo...
example 3
[0059] A 0.6% solution of polycaprolactone dissolved in methylene chloride was prepared at room temperature. The solution was sprayed onto a Sulzer Intratherapeutics nitinol Protege model endovascular stent (6 mm.times.20 mm) using a semi-automated nebulizer apparatus. The nebulizer spray system provided a means of rotating and traversing the length of the stent at a controlled rate. The traversing component of the apparatus contained a glass nebulizer system that applied nebulized polycaprolactone solution to the stent at a rate of 3 ml per minute. Once applied, the 10 mg polymer coating was "reflowed" by application of 60.degree. C. heated air for approximately 5 seconds. The process of reflowing the polymer provides better adherence to the stent surface. A drug-loaded polymer coating can be provided using this technique by first preparing a 1%-20% cerivastatin / polymer solution in methylene chloride with subsequent application to the stent surface using the same nebulizer coating...
example 4
[0060] A 1% solution of uncured two-part silicone rubber dissolved in trichloroethylene was applied to a "Protege" nitinol stent in the manner described in Example 3. The coated stent was dried at room temperature for 15 minutes to allow the trichloroethylene to evaporate. Once 10 mg of silicone was coated onto the stent, the composite device containing both uncured polymer and nitinol was heated in a vacuum oven for a period of four hours in order to crosslink the silicone coating. After the coated stents were removed from the oven and allowed to cool for a period of 1 hour, cerivastatin was loaded into the silicone coating by the following method. Three mg of cerivastatin was dissolved in 300 .mu.l of methylene chloride at room temperature. A volume of 100 .mu.l of methylene chloride was applied to the silicone coating of each stent in dropwise fashion. In this manner, each stent was loaded with 1 mg cerivastatin, for a final concentration of 10% w / w. The crosslinked silicone abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com