Structure of quinoxalinone derivatives as aldose reductase inhibitor, preparation method and use
A technology of structural formula and raw materials, applied in the prevention and/or treatment of diabetic complications, structure and preparation field of aldose reductase inhibitors, can solve problems such as insufficient activity intensity, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
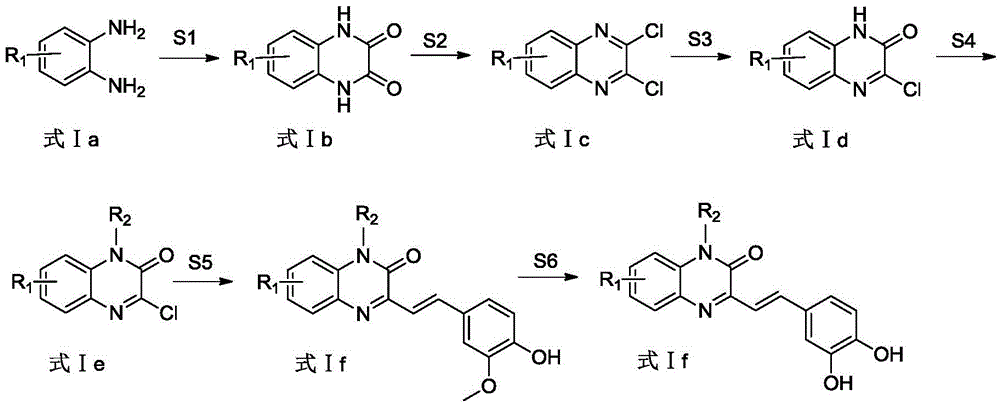
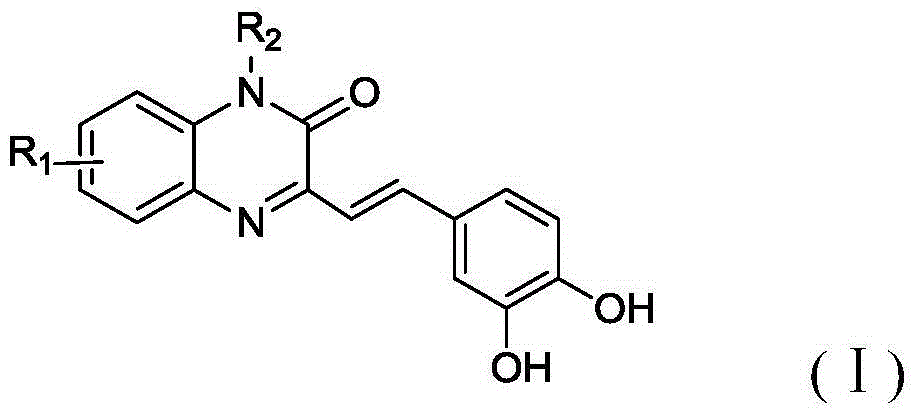
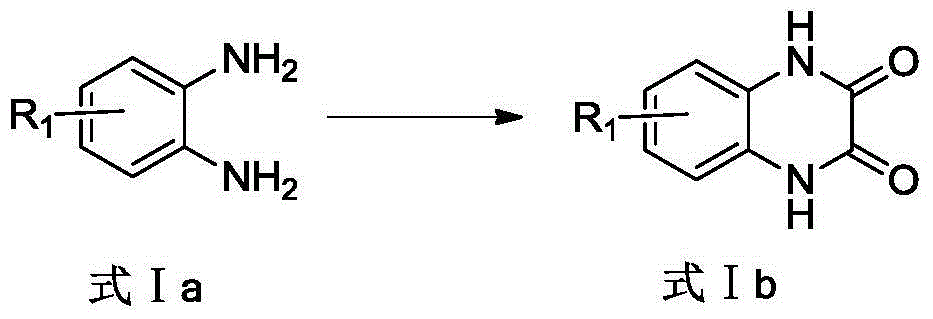
[0050] see figure 1 , the embodiment of the present invention provides a class of a class of quinoxalinone derivatives as the structure of an aldose reductase inhibitor and a preparation method thereof, which includes the following steps:
[0051] S2: the chlorination reagent used in the reaction can also be phosphorus oxychloride, phosphorus oxychloride, etc. in addition to thionyl chloride, and the reaction temperature is 40°C-50°C;
[0052] S3: The alkali used in the reaction, in addition to lithium hydroxide, can also be sodium hydroxide, potassium hydroxide, potassium carbonate, etc., and the reaction temperature is 55°C-75°C;
[0053] S5: the catalyst used in the reaction, except palladium acetate and tri-o-methylphenylphosphine, can also be tetrakistriphenylphosphine palladium, palladium acetate and triphenylphosphine, palladium chloride and triphenylphosphine, Palladium chloride and trimethylphenylphosphine etc.; The alkali used in the described reaction, except tr...
Embodiment 1
[0055] Example 1: Preparation of 2-(3-(3,4-dihydroxystyryl)-2-oxoquinoxaline-1(2H)-alkyl)acetic acid
[0056] (compound 1)
[0057]
[0058] Put 10.8g (100mmol) of o-phenylenediamine and 13.5g (150mmol) of oxalic acid into a round bottom flask, then add 100mL of water and 10mL of concentrated hydrochloric acid, heat, stir and reflux for 6 hours, and after returning to room temperature, filter the reaction solution, Repeated washing with water and drying gave quinoxaline-2,3(1H,4H)-dione (colorless crystals, yield 96%, 15.6g): 1 H NMR (400MHz, [D 6 ]DMSO): δ 7.063 (d, 2H, J = 6.4Hz), 7.114 (d, 2H, J = 6.4Hz), 11.893 (s, 2H).
[0059] Add 8.1g (50mmol) of quinoxaline-2,3(1H,4H)-dione, 11.9g (100mmol) of thionyl chloride, and 5mL of DMF into a round-bottomed flask containing 50mL of dichloromethane, and heat to reflux Stir until the starting material spot disappears as monitored by TLC. The reaction solution was cooled to room temperature, and poured into ice water careful...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com