Stent with sheath and metal wire and methods
a technology of metal wire and stent, which is applied in the field of stent with sheath and metal wire and methods, can solve the problems of injuring the blood vessel, affecting the thrombosis of the patient, so as to reduce the development of thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0101]The instant application is divided into a number of labeled sections which generally include, in order, descriptions of apparatuses (e.g. porous structures, stents, etc.), materials and methods for manufacturing the apparatuses, the usage of pharmaceuticals with the apparatuses and methods of using the apparatuses. It should be understood that the section headings are for clarity only, and are not intended to limit the subject matter described therein. Furthermore, some of the subject matter described in a particular section may belong in more than one section and therefore, some of the material could overlap between sections.
Overview of Exemplary Enhanced Stent Apparatus
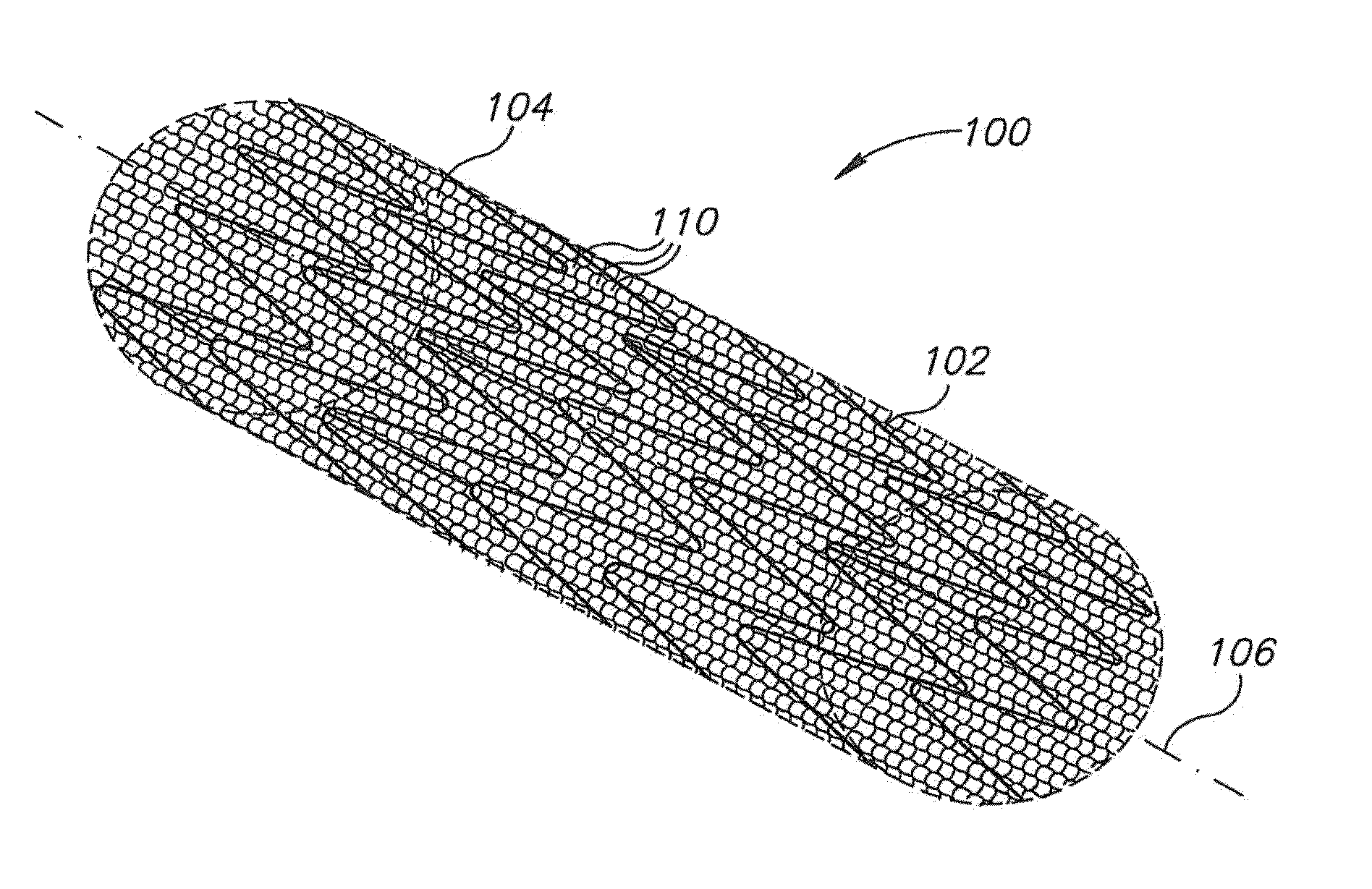
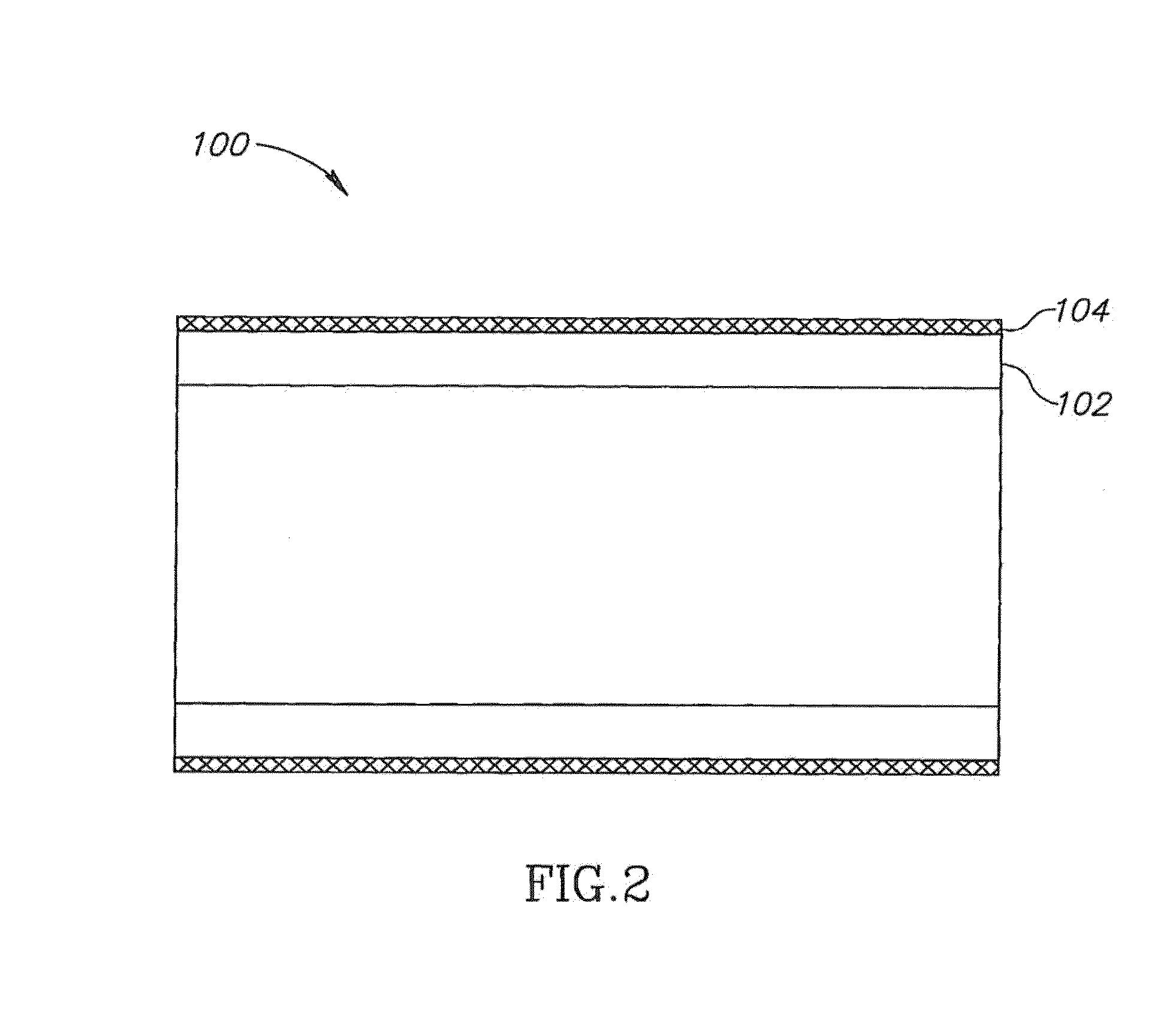
[0102]In an exemplary embodiment of the invention, an apparatus is provided which includes a porous structure and, optionally, an underlying support element, such as a stent (wherein underlying means the porous structure is between the support element and a lumen wall).
[0103]In some exemplary embodiments of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com