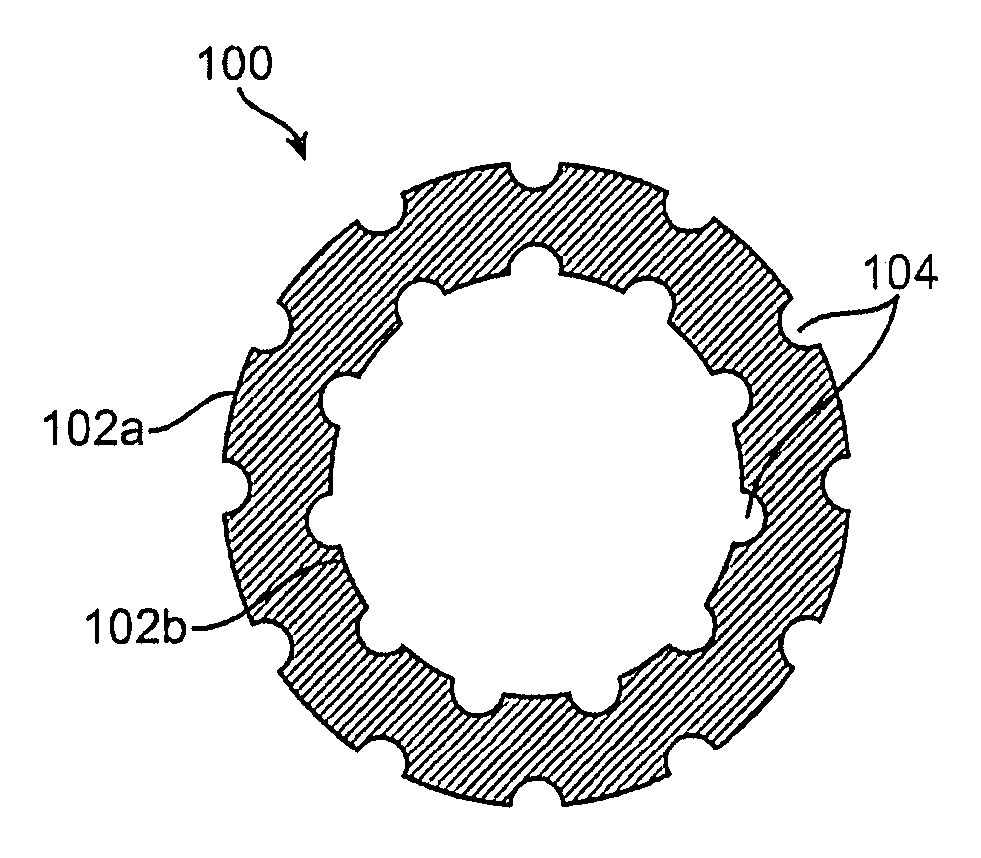
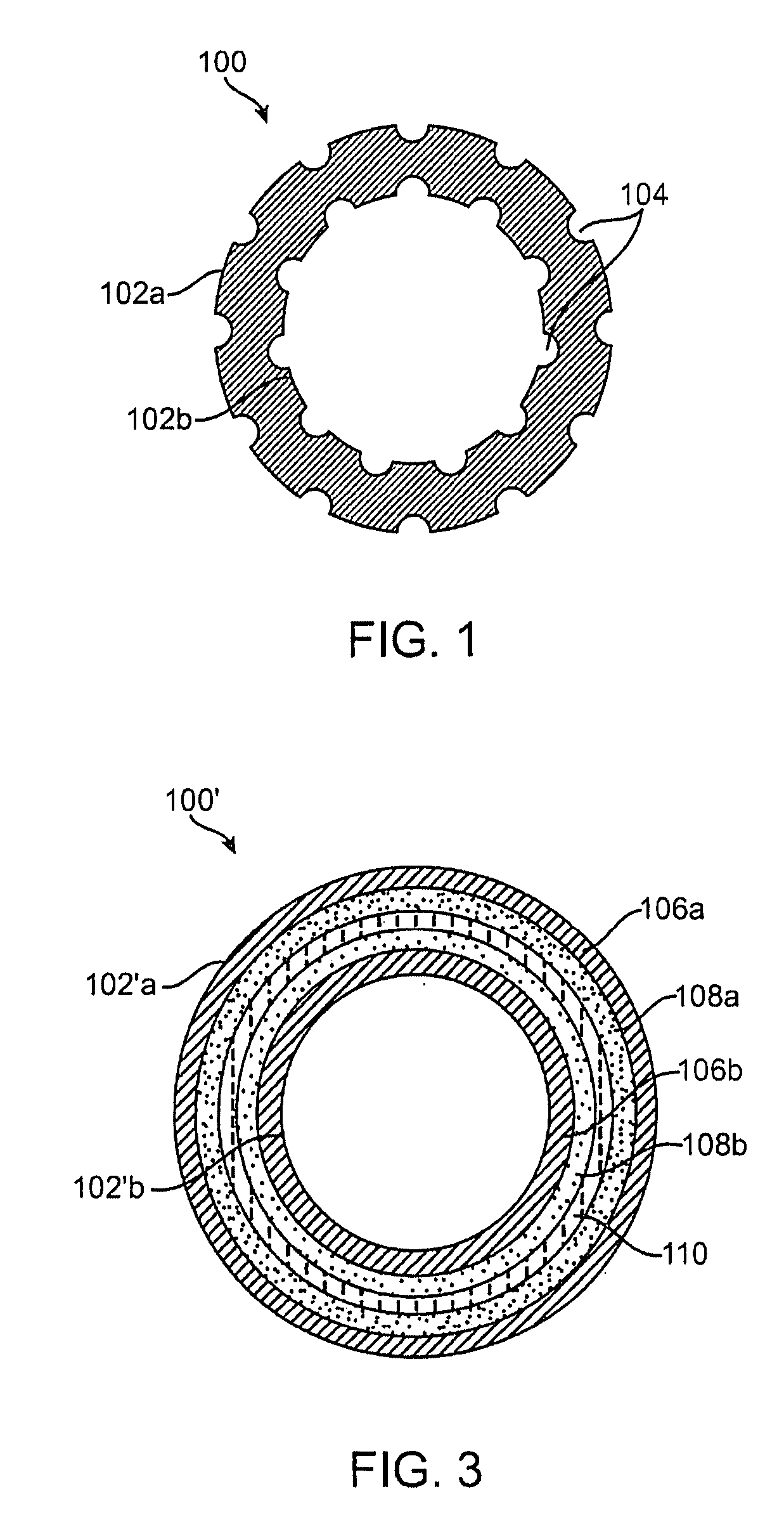
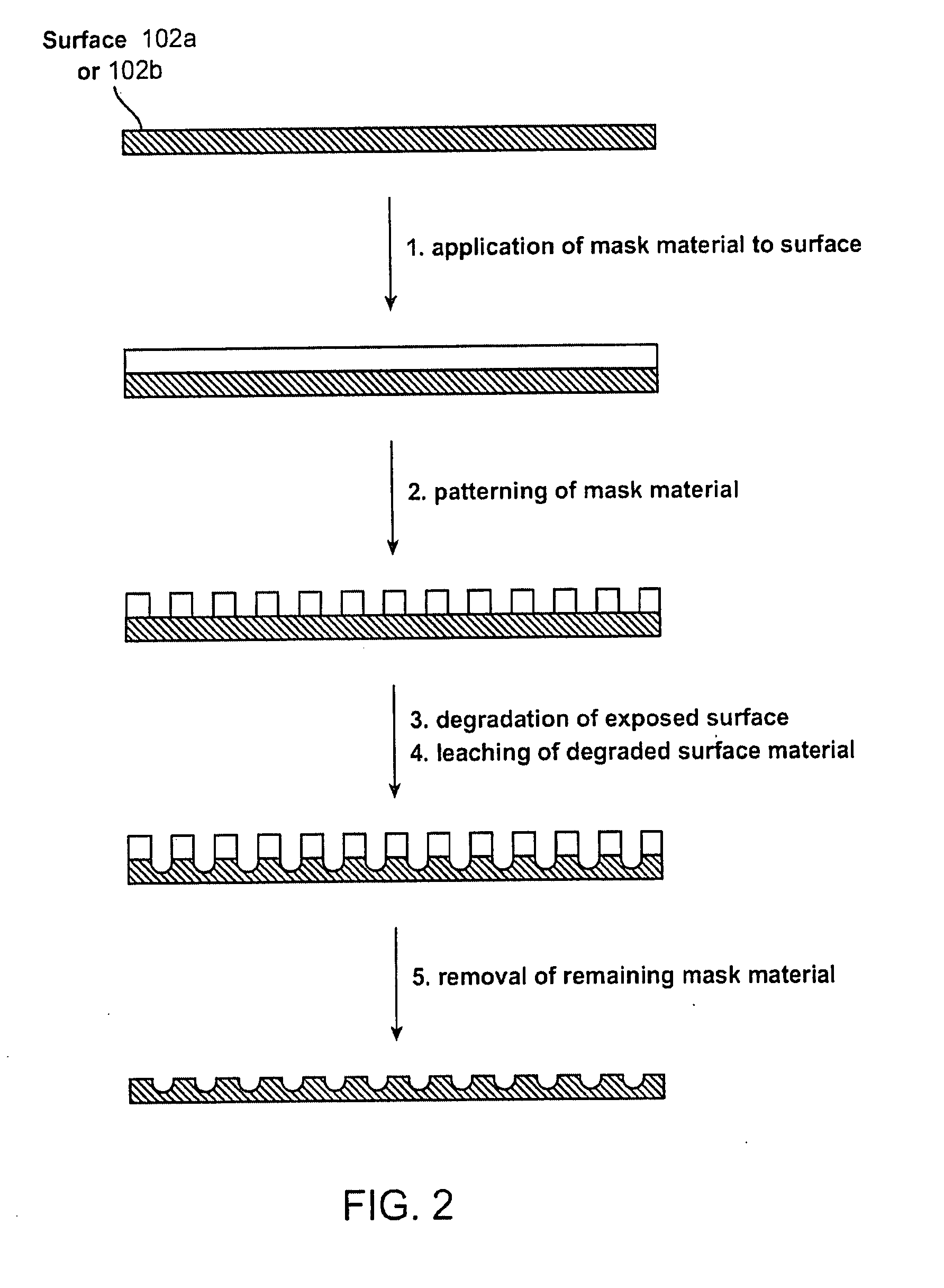
Implantable article, method of forming same and method for reducing thrombogenicity
a technology of implantable devices and polymer materials, applied in the field of implantable devices, can solve the problems of reducing the thrombogenicity of polymer materials used in the production of implantable devices, unable to overcome problems, and unable to achieve the effect of reducing the thrombogenicity of implantable devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0097] For the following examples, each polymer was first dissolved in chloroform. Nano-sized salt particles were ground and sieved, and then dispersed in the polymer solution with constant stirring until the particles were visually uniformly dispersed. The polymer concentration was chosen such that it had sufficiently high viscosity to maintain a stable dispersion. The dispersion was then cast as a film of required thickness using a coater. The film was dried in an oven at 37° C., and then left at room temperature for several days in a dry environment. The dried films were immersed in water for 14 days, with constant exchange of the water. The salt nano-particles were thus leached out, and the resulting film was dried again at 37° C. and at room temperature.
[0098] Control films were prepared as pure polymer films without any surface modification.
[0099] PLLA and PLGL films having nano-craters in the surface were obtained by leaching out incorporated nano-particles of NaCl, as indi...
example 2
[0102] In another example of a method for modifying a surface of a polymer for implantation within a patient body, porogen leaching of surfaces may be utilized to yield a surface which enhances endothelial cell growth over a defined range of surface features. In this particular example, surface pores were created by filling polymers such as Poly caprolactone (PCL), Poly L-lactide (PLLA), Poly (lactide-co-glycolide), etc. (although any of the other suitable polymers described herein may be utilized) with leaching agents of sugar and gelatin.
[0103] The sugar and gelatin particles ranged in size from 20 to 90 microns in diameter (although particles as small as 5 microns may also be utilized) where the average particle sizes typically ranged from 20, 45, and 90 microns. The leaching agents were added in concentrations ranging from 1 to 10% by weight in the polymer. More particularly, the leaching agents were added in concentrations ranging from 1%, 5%, and 10% by weight in the polymer....
example 3
[0109] As mentioned above, chemicals such as sodium hydroxide may be used to dissolve PLA in regions unprotected by an alkali-resistant mask material where the dissolved material may be rinsed away in water to form nano-craters. In another example, the polymer surface may be first irradiated prior to etching with the sodium hydroxide to enhance the etching process.
[0110] In this example, samples of PLGA, PCL, and PLLA (other suitable polymers described above may alternatively be utilized) were first irradiated with an electron beam and then etched using the sodium hydroxide, as described above, for a period of 16 hours to create surface features. The average surface roughness of the samples was measured using an atomic force microscope (AFM) and the etched samples were then exposed to endothelial cells. Growth was quantified over a period of 15 days and the irradiated and etched samples were compared to control samples after 4 days, 8 days, and 15 days. Table 2 shows a comparison o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com