Use of Activin A in human embryo stem cell trophoblast-free cell culture
A technology of human embryonic stem cells and trophoblast cells, applied in the field of biomedicine, can solve the problem that the in vitro culture conditions of human embryonic stem cells are not optimal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 , Culture of human embryonic stem cells
[0029] 1.1. Culture of human embryonic stem cells
[0030]The Non-CM formulation used in this example is as follows: 80% by weight of DMEM / F12 culture fluid, 20% by weight of serum substitute, 1mML-glutamic acid, 0.1mM β-mercaptoethanol, 1% by weight of non-CM Essential amino acids (Invitrogen, catalog number: 11140050).
[0031] The culture method of H1 human embryonic stem cells is as follows: the culture bottle is treated with matrigel glue, and a sterile medium is filled. Inoculate H1 human embryonic stem cells in culture medium, place them in a carbon dioxide incubator, and store them at 37°C in 5% volume ratio of CO 2 Cultured for 6 days.
[0032] The experiments were divided into 4 groups:
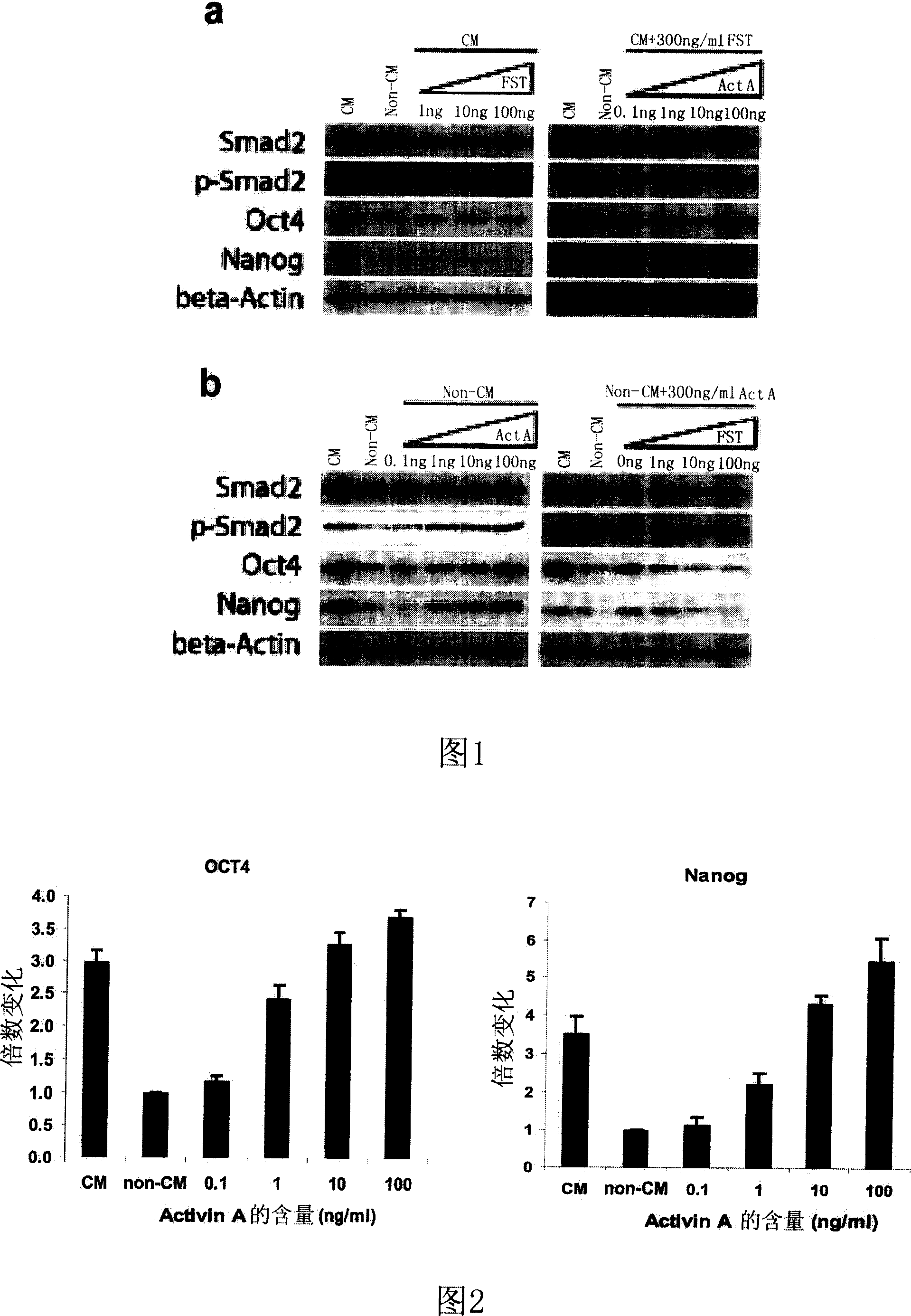
[0033] Group 1: CM culture, Non-CM culture, and FST culture with concentrations of 1ng / ml, 10ng / ml and 100ng / ml in CM;
[0034] Group 2: CM culture, Non-CM culture, and CM containing 300ng / ml FST added Activin A at a c...
Embodiment 2
[0047] Example 2 , quantitative RT-PCR analysis
[0048] According to the method of Noaksson, K. et al. (Noaksson, K., et al. Stem Cells. 2005, 23, 1460-7), RNA isolation and quantitative RT-PCR analysis were performed on the cells cultured for 6 days in Example 1.
[0049] Total RNA was prepared by RNAeasy kit (Qiagen) and used as a template for RT-PCR. Real-time quantitative PCR was performed on MyiQ real-time quantitative PCR detection system (Bio-Rad) using SYBR GreenI-based PCR Master Mix (Bio-Rad).
[0050] PCR primers are:
[0051] Upstream of Oct4: 5'-GAGAAGGATGTGGTCCGAGTGTG-3';
[0052] Downstream: 5'-CAGAGGAAAGGACACTGGTCCC-3';
[0053] Nanog upstream: 5'-TGAACCTCAGCTACAAACAGGTG-3';
[0054] Downstream: 5'-AACTGCATGCAGGACTGCAGAG-3'.
[0055] The PCR conditions are all: 94° C. for 30 seconds, 66° C. for 30 seconds, and 72° C. for 30 seconds for 35 cycles.
[0056] Each experiment was repeated at least 3 times. The experimental value for each ge...
Embodiment 3
[0058] Example 3 , the formation of teratoma
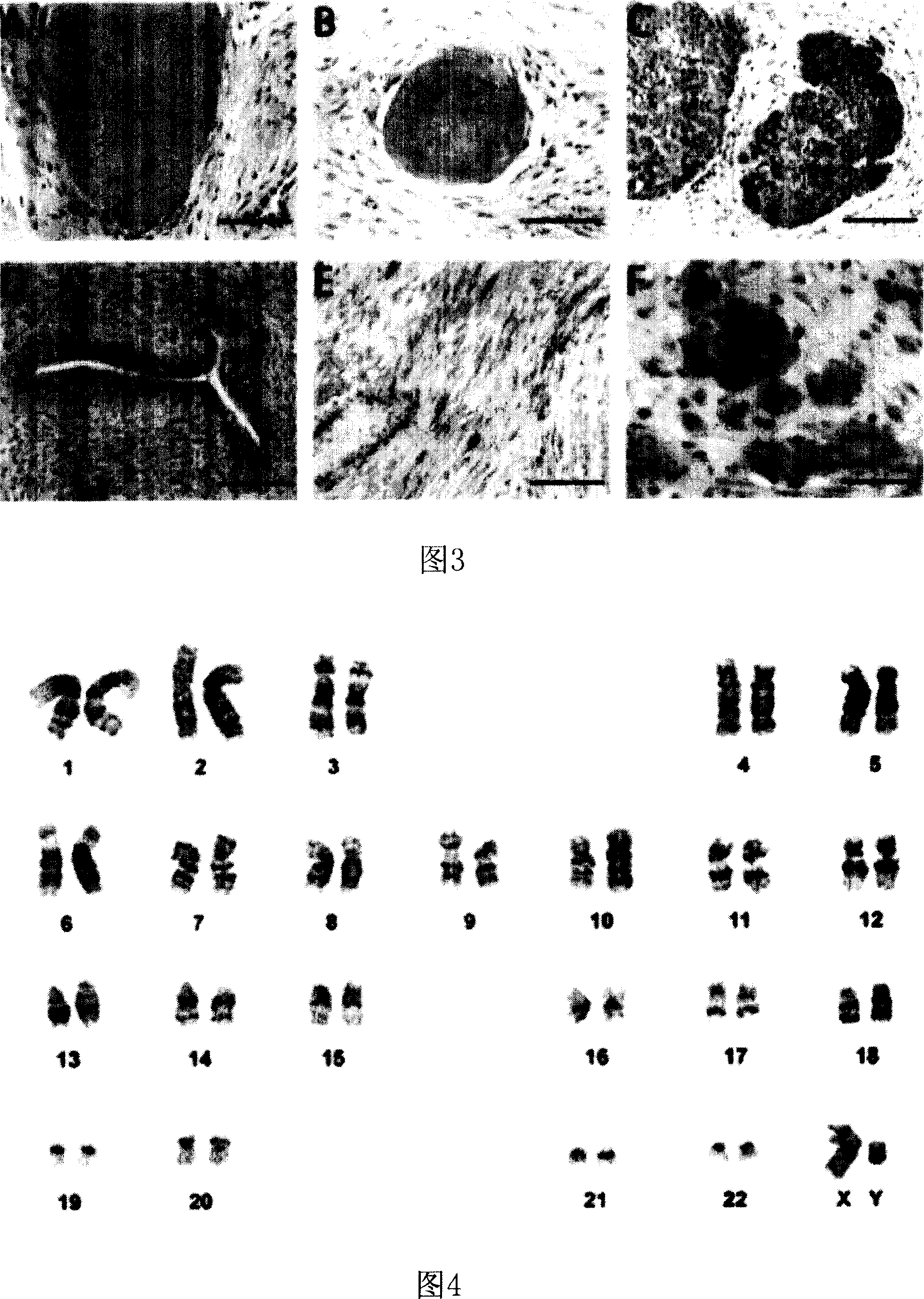
[0059] According to the method of step 1.1, culture H1 human embryonic stem cells with Non-CM containing 5ng / ml Activin A, passage once a week, treat with 1mg / ml Dispase protease at 37°C for 30 minutes during passage, and transfer to a new culture flask.
[0060] After 10 generations of culture, approximately 5×10 6 Cells were injected intramuscularly into NOD / SCID mice (Taconic). After 4-6 weeks, the tumor was removed, stained with hematoxylin-eosin and immunohistochemical staining according to the method in the literature (Fan G., et al.FEBS Lett.2000, 467(1):7-11) , and the result is shown in Figure 3.
[0061] The staining results in Figure 3 showed that these teratomas contained tissues derived from ectoderm, mesoderm, and endoderm. Among them, A is nerve tissue, B is bone tissue, C is liver tissue, A, B, and C are stained with HE; D is anti-pan-keratin antibody staining, showing the existence of ectodermal tissue epider...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com