Human umbilical cord and placenta protection solution mycoplasma detection primer pair, kit and detection method thereof
A technology for detection kits and protective solutions, which is applied in biochemical equipment and methods, microbe measurement/inspection, DNA/RNA fragments, etc. It can solve problems such as inaccurate experimental results, unfavorable stem cell culture, and long culture time. Achieve high sensitivity, prevent mycoplasma from entering the cell culture room, and take a short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of Human Umbilical Cord and Placenta Protection Liquid Mycoplasma Detection Kit
[0045] 1. Primer synthesis
[0046] The following primer pairs were artificially synthesized:
[0047] Upstream primer Myco-F: 5'-ggcgaatgggtgagtaacacg-3'; (SEQ ID NO.1)
[0048] Downstream primer Myco-R: 5'-cggataacgcttgcgacctatg-3'. (SEQ ID NO.2)
[0049] Primers were prepared with ultrapure water and diluted to 2.5 μM, and stored at -20°C for later use.
[0050] 2. Preparation of positive control
[0051] In the process of stem cell culture, the cells that are confirmed to be contaminated by mycoplasma by the separation and culture method, use a Pasteur pipette or a pipette to absorb 1 mL of the cell culture supernatant, and take 3 μL as the sample to be tested, and then amplify by PCR using the primer pair in the above step After that, it was analyzed by agarose gel electrophoresis with a mass percentage of 2%, and a clear band was obtained at 400-500 bp, whi...
Embodiment 2
[0058] Example 2 Application of Human Umbilical Cord and Placenta Protection Liquid Mycoplasma Detection Kit
[0059] Samples were tested using the kit prepared in Example 1.
[0060] 1. Obtaining samples to be tested
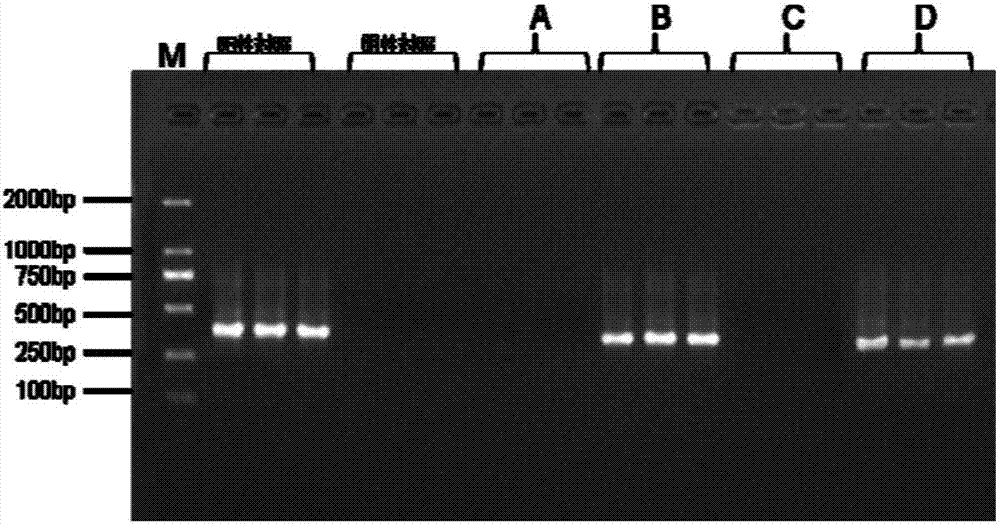
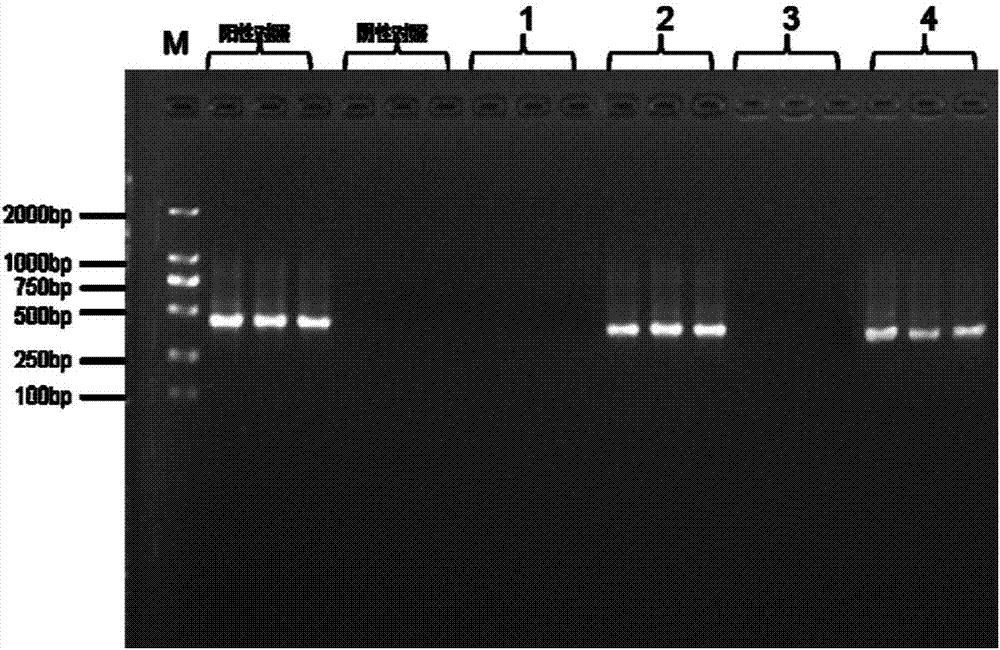
[0061] According to the aforementioned method, take three tubes of the umbilical cord protection solution not polluted by mycoplasma as the test samples of group A; three tubes of the umbilical cord protection solution polluted by mycoplasma as the test samples of group B; Three tubes were used as test samples of group C; three tubes of the placenta protection solution contaminated by mycoplasma were used as test samples of group D.
[0062] 2. PCR amplification
[0063] 1. Use groups A, B, C, and D as samples to be tested, and add the following reagents into the PCR reaction tube according to the amplification system:
[0064] 2×power Taq PCR MasterMix 5μL;
[0065] Upstream primer 2.5μM 1μL (2.5μM);
[0066] Downstream primer 2.5μM 1μL (2.5μM);
[0067]...
Embodiment 3
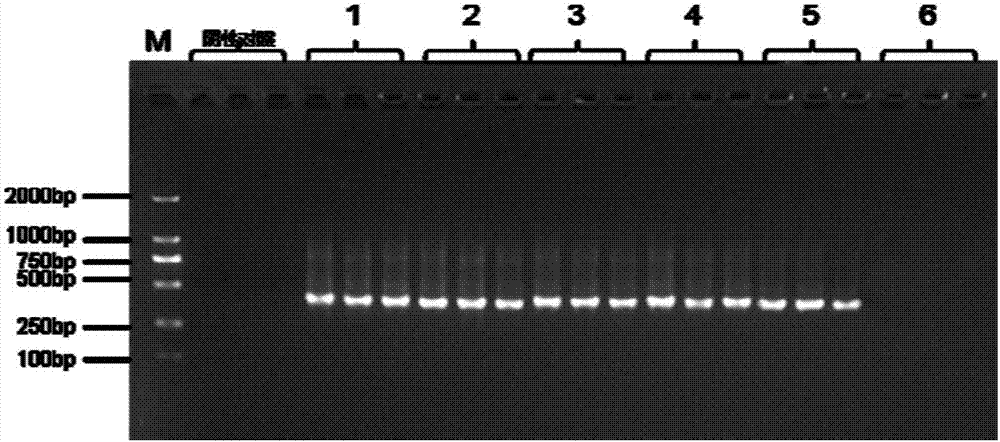
[0072] Example 3 Sensitivity Detection of Human Umbilical Cord and Placenta Protection Liquid Mycoplasma Detection Kit
[0073] Samples were tested using the kit prepared in Example 1.
[0074] 1. Obtaining samples to be tested
[0075] In the process of stem cell culture, the cells that are confirmed to be contaminated by mycoplasma by the separation and culture method, use a Pasteur pipette or a pipette to absorb 1 mL of the cell culture supernatant, and take 3 μL as the sample to be tested, and then amplify by PCR using the primer pair in the above step After that, it was analyzed by agarose gel electrophoresis with a mass percentage of 2%, and a clear band was obtained at 400-500bp, and the product was recovered by cutting the gel.
[0076] The PCR reaction system is PCR reagent 2×power Taq PCR MasterMix 5 μL, upstream primer 2.5 μM 1 μL, downstream primer 2.5 μM 1 μL, sample to be tested 3 μL;
[0077] The PCR amplification program is: 94° C. for 3 min; 94° C. for 30 s,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com