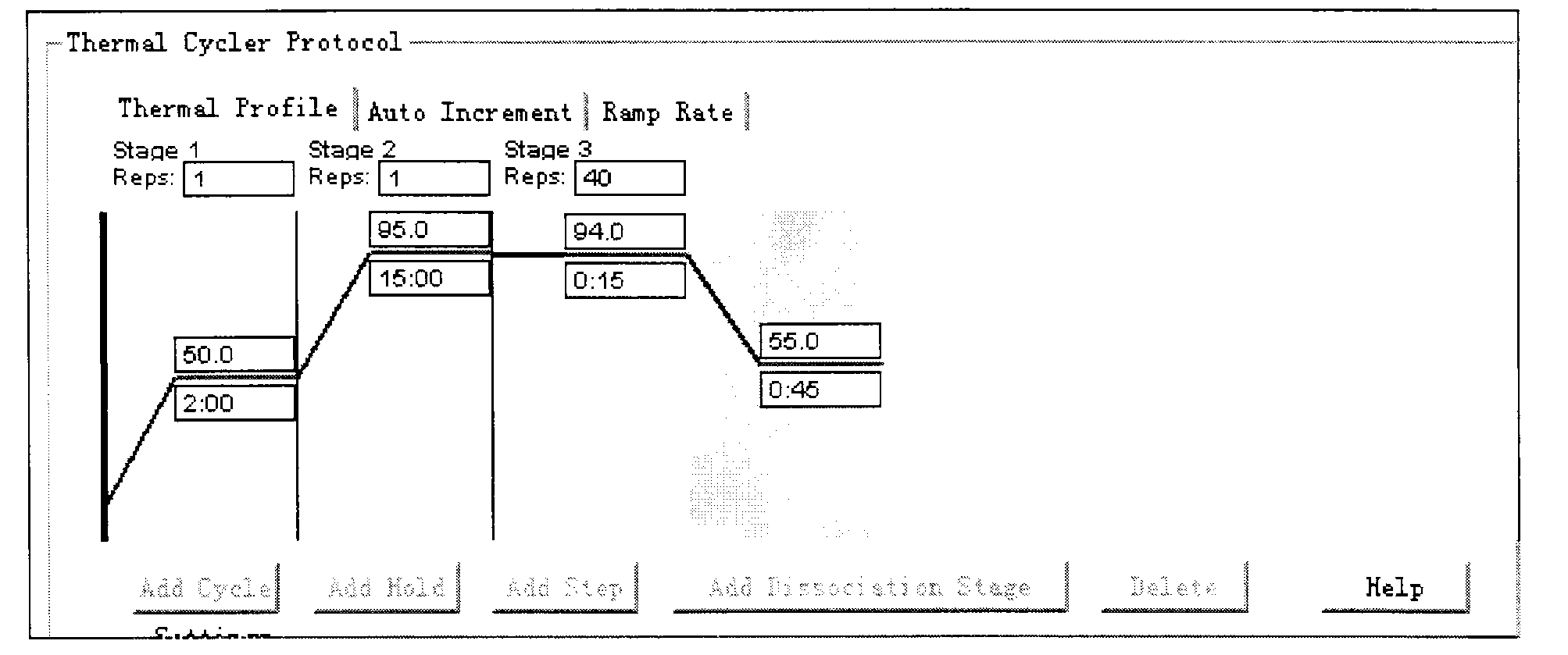
Klebsiella pneumoniae nucleic acid detection kit (PCR-fluorescent probe method)
A technology of Klebsiella pneumoniae and oligonucleotide probes, applied in the fields of fluorescence/phosphorescence, microbial measurement/testing, biochemical equipment and methods, etc., can solve problems such as atypical clinical manifestations and difficult diagnosis, and achieve Avoid false positive results, fast detection speed, and the effect of preventing contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: KP kit detection method of Klebsiella pneumoniae
[0038] (1) Specimen collection, transportation and preservation
[0039] Specimens were collected by clinicians according to the actual situation. Testable specimens include throat swabs, nasopharyngeal secretions, or sputum. The collection methods are as follows: ① Pharyngeal swab: take the pharyngeal secretions with a cotton swab, put the cotton swab into a sterile glass tube, and seal it for inspection; ② nasopharyngeal secretions: absorb the nasopharyngeal secretions under negative pressure, seal them Submit for inspection; ③Human sputum specimen: Aseptically collect and instruct the patient to cough up sputum (not containing saliva) from the deep part of the respiratory tract, put it in a 40ml sterile plastic container, cover the container, and seal it for inspection. Specimens can be used for testing immediately, or can be stored at -70°C for testing, and the storage period is 6 months. Specimens ...
Embodiment 2
[0063] Example 2: Preparation and use of quality control products in the Klebsiella pneumoniae nucleic acid detection kit
[0064] The quality control products in the Klebsiella pneumoniae nucleic acid detection kit include positive quality control products and negative quality control products, which are used for quality control in clinical trials. KP positive quality control products are directly added to the machine after centrifugation, and negative quality control products are operated The method is the same as that of the sample to be tested.
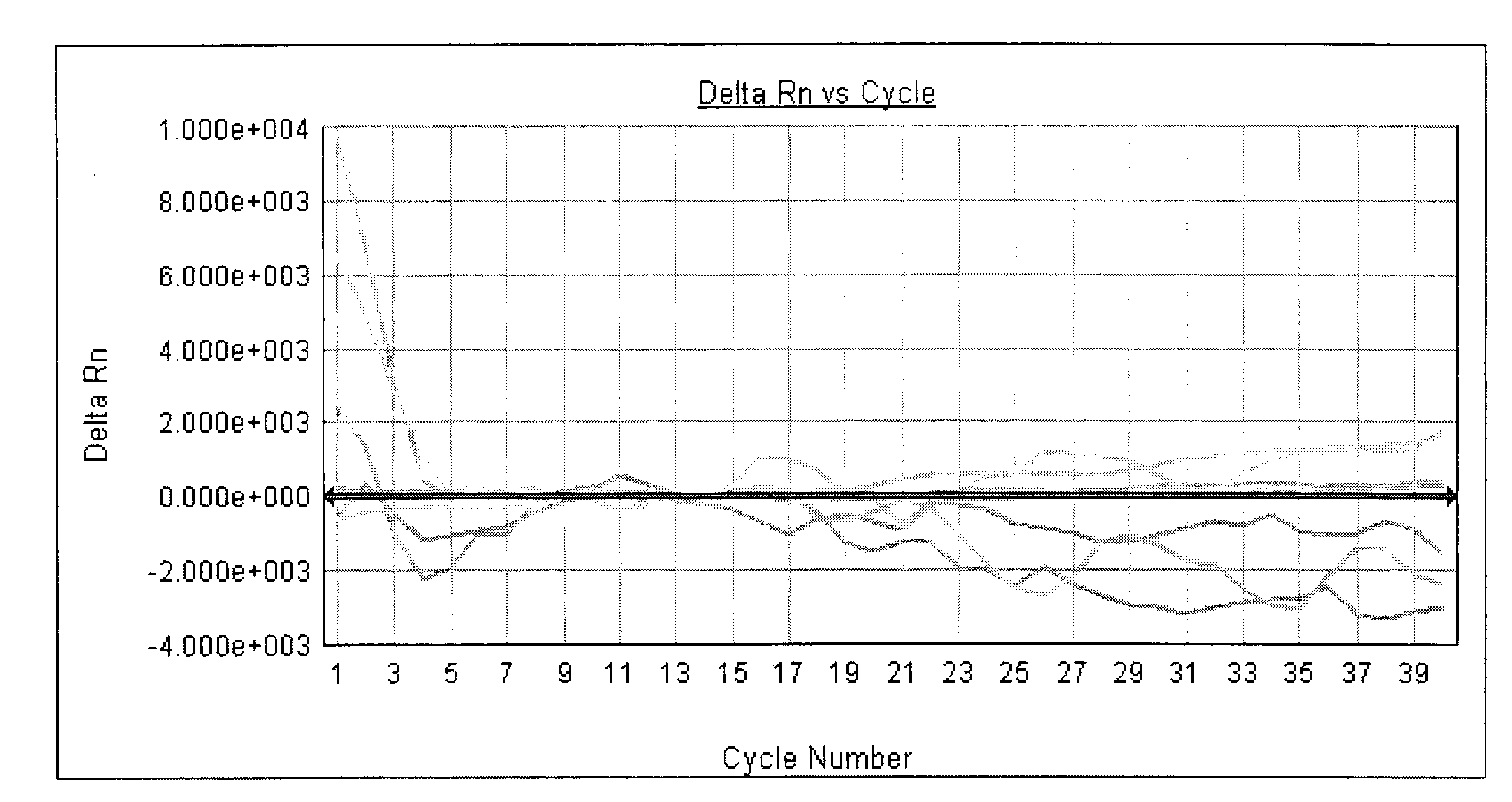
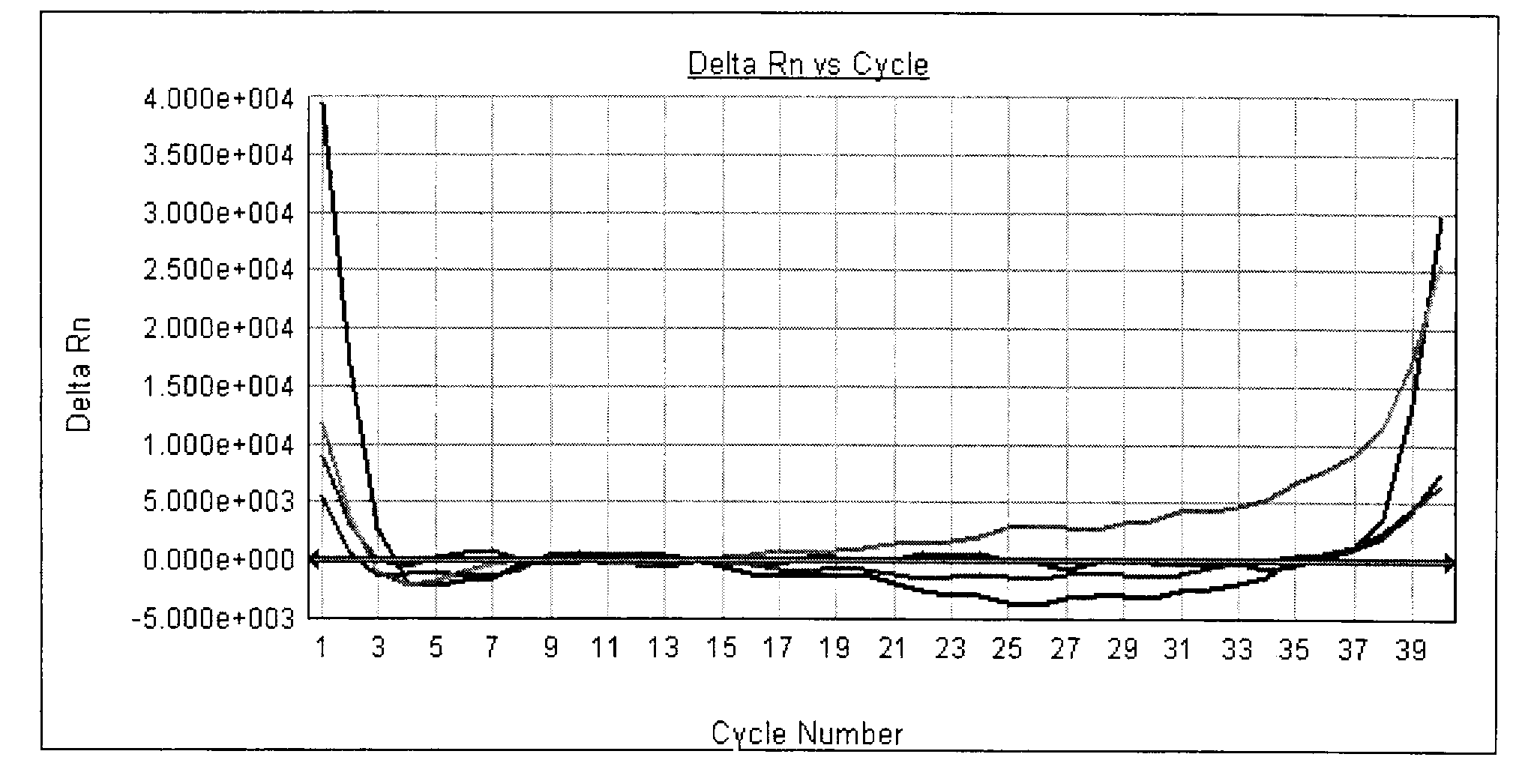
[0065] Quality control standard: It is required to meet the following conditions in one experiment at the same time - the positive quality control product is positive (see attached Figure 5 ), the negative quality control was negative (see attached Image 6 ); otherwise, the result is invalid and needs to be tested again.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com