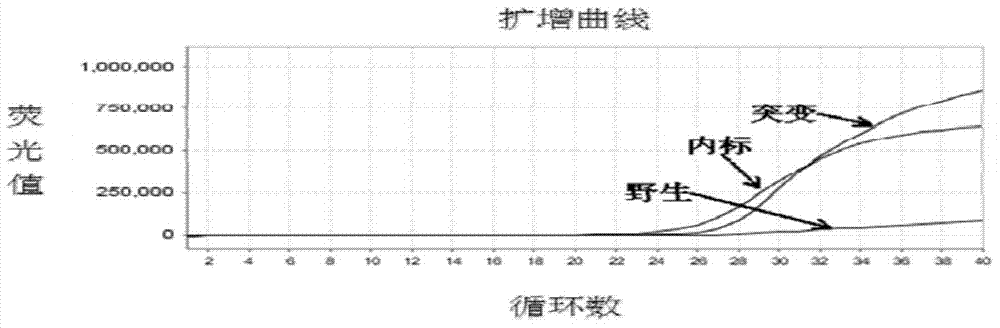
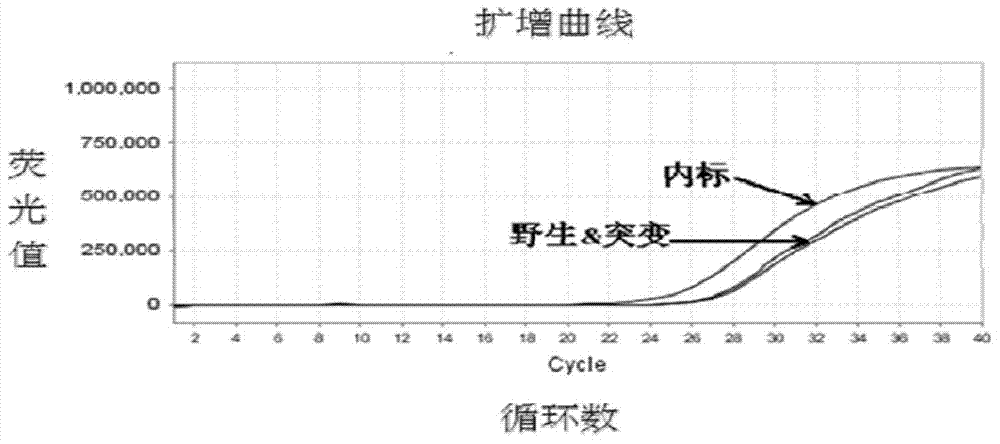
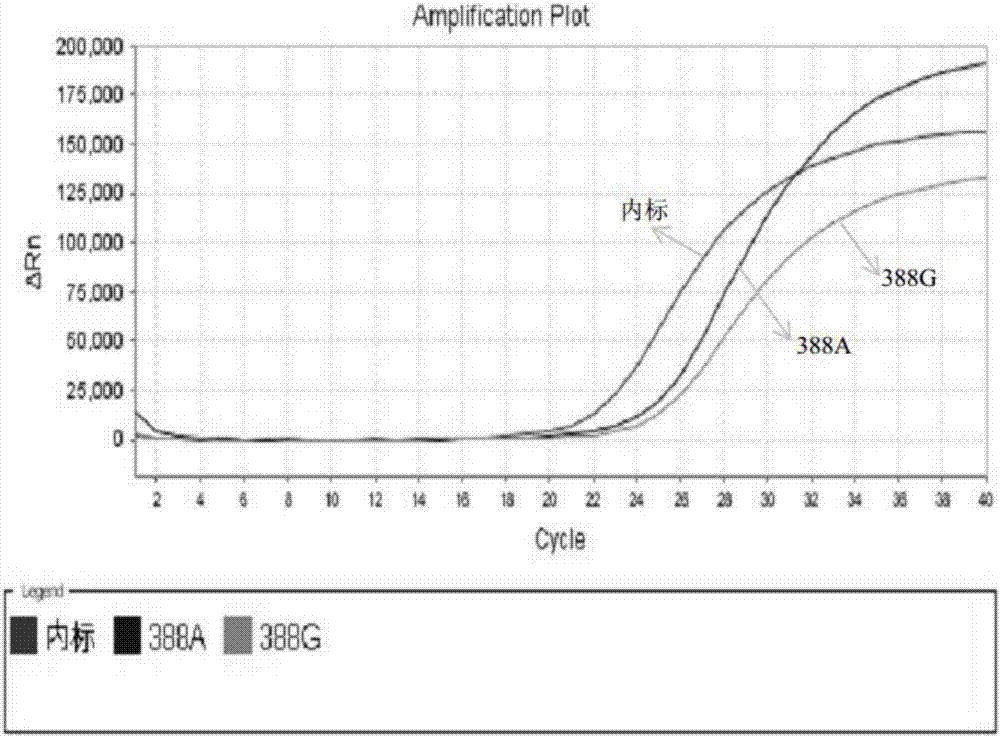
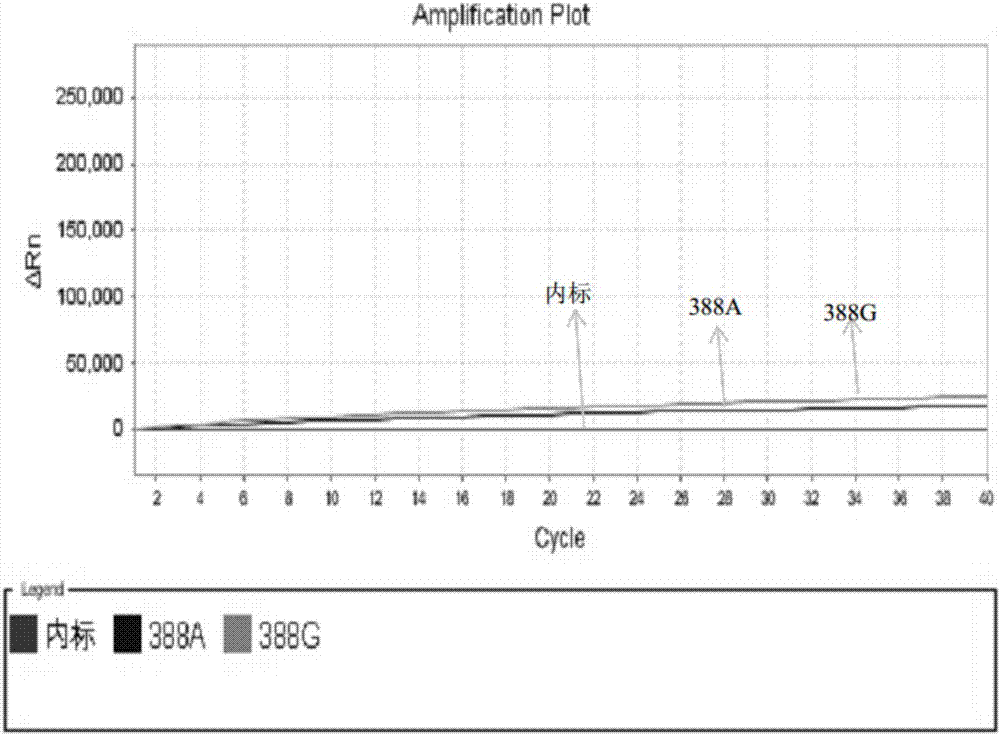
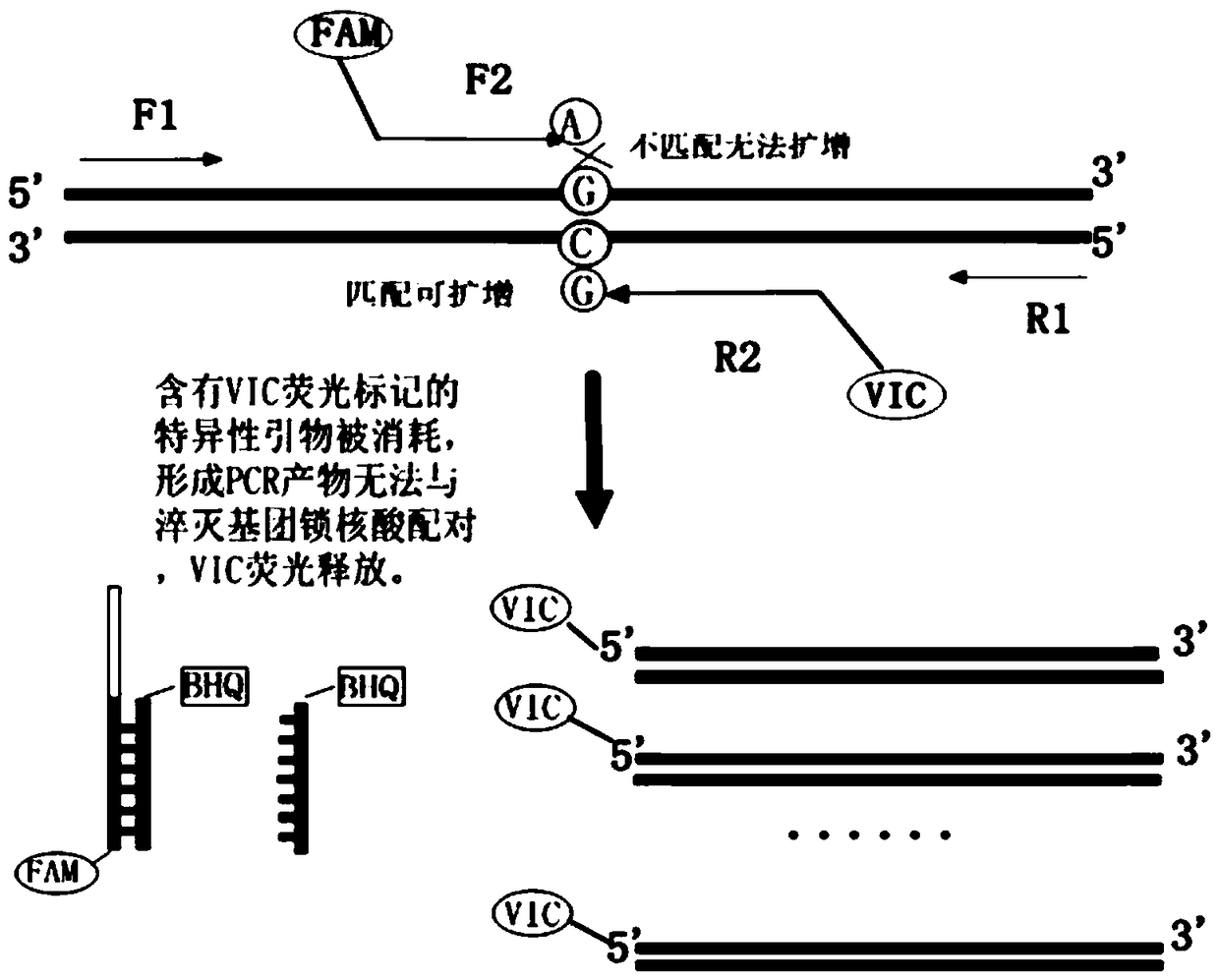
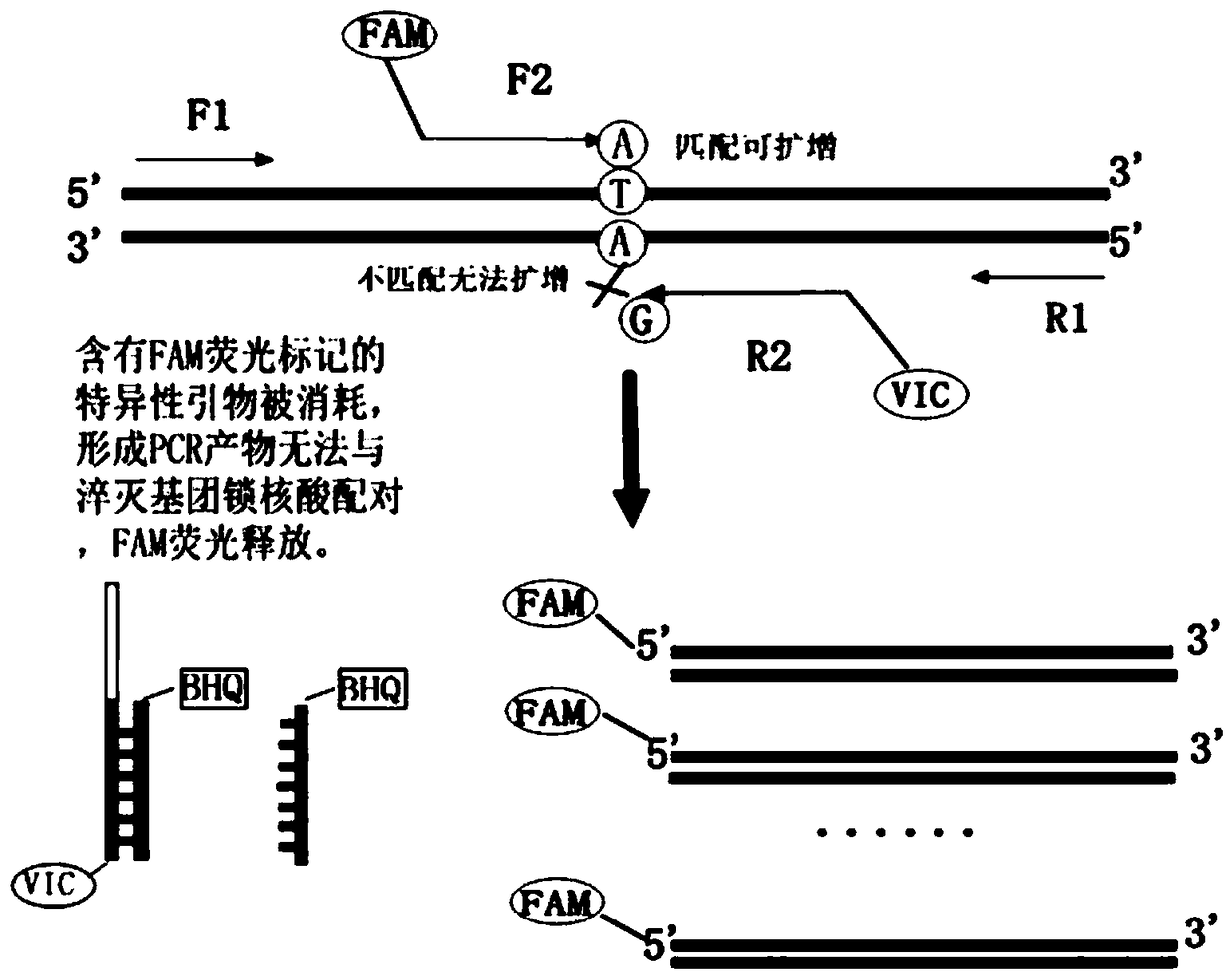
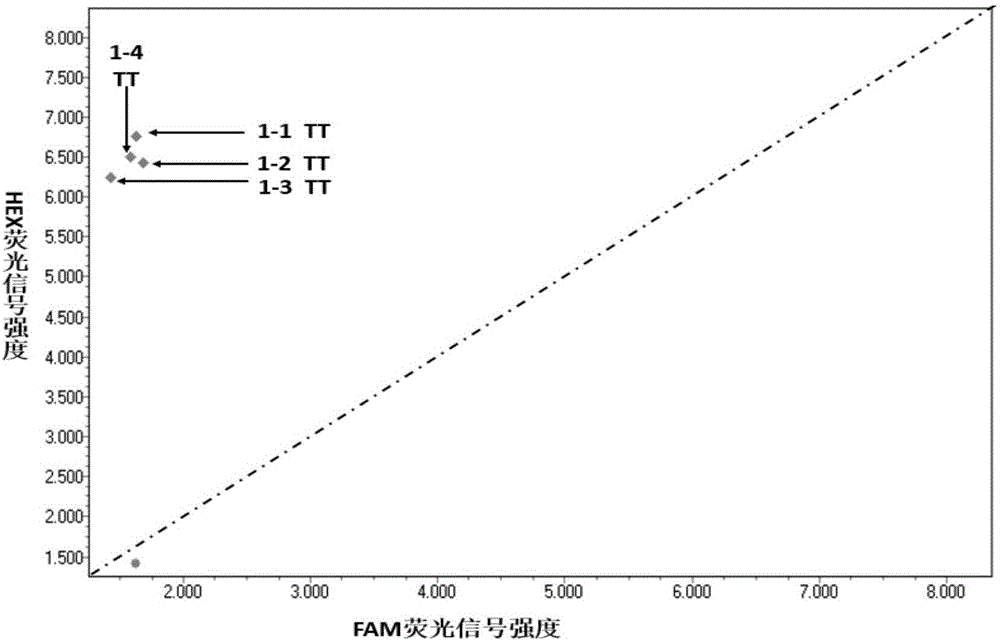
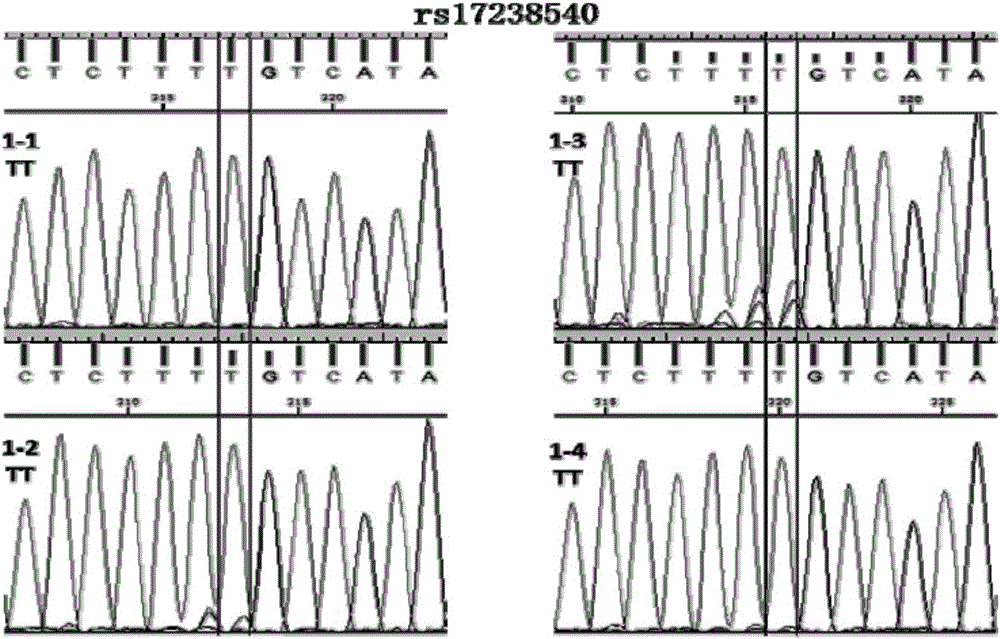
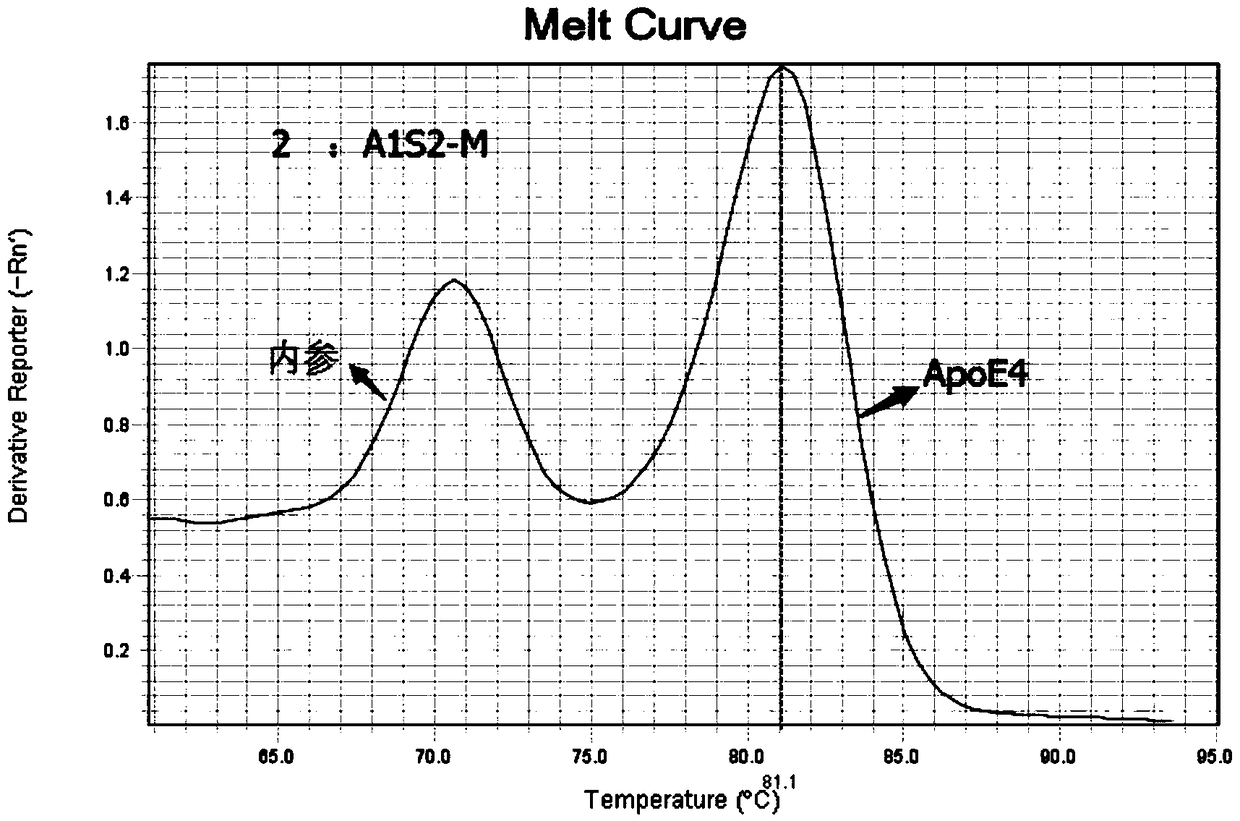
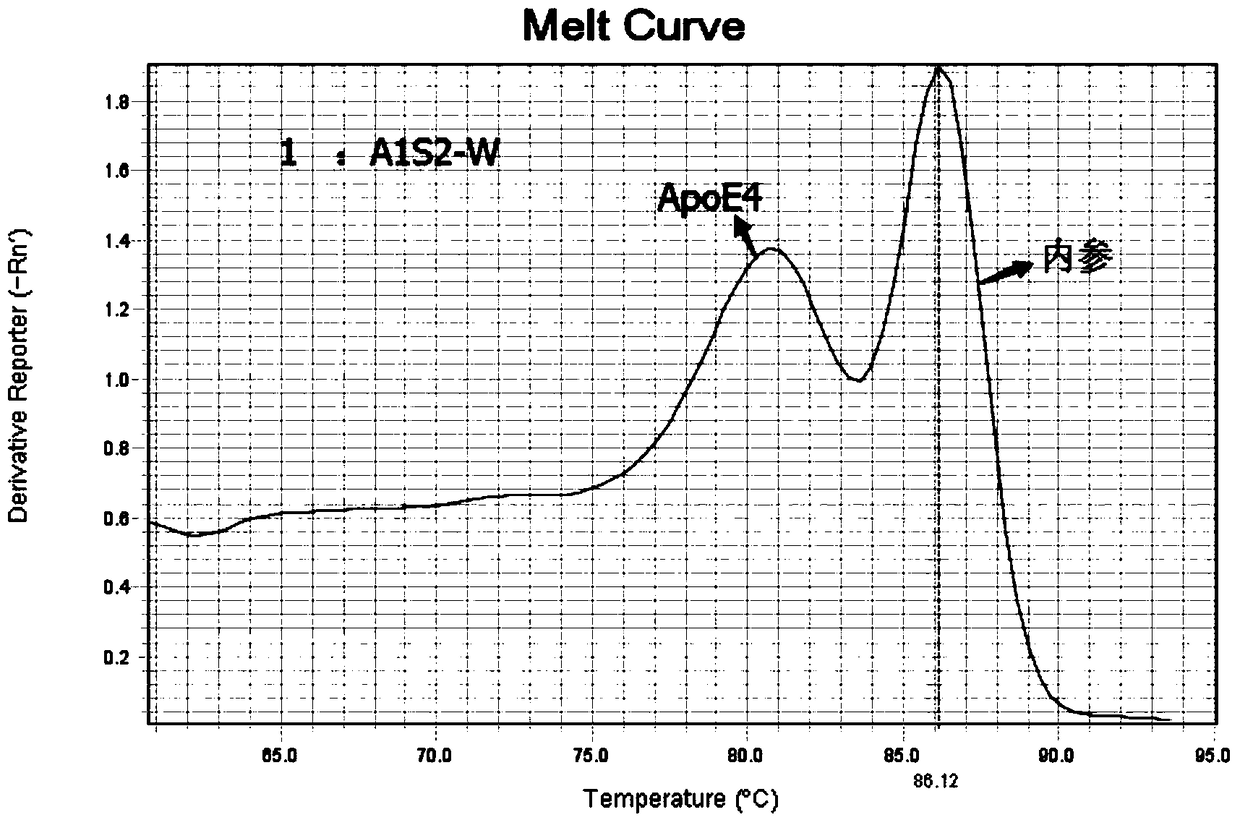
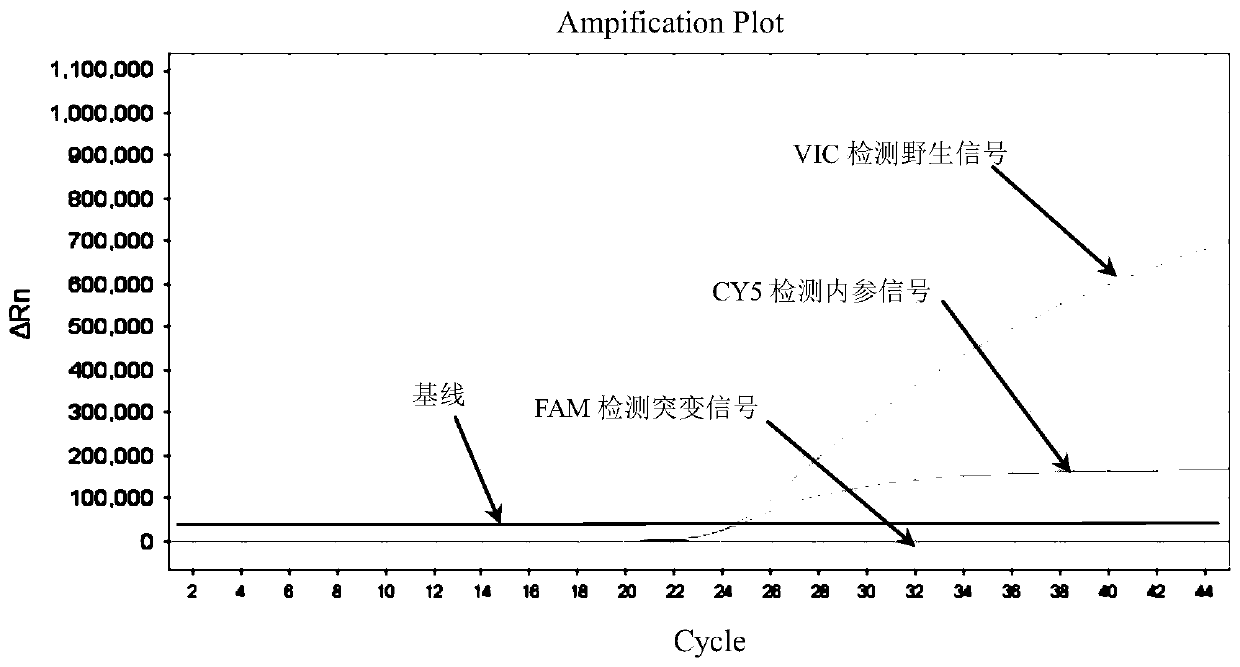
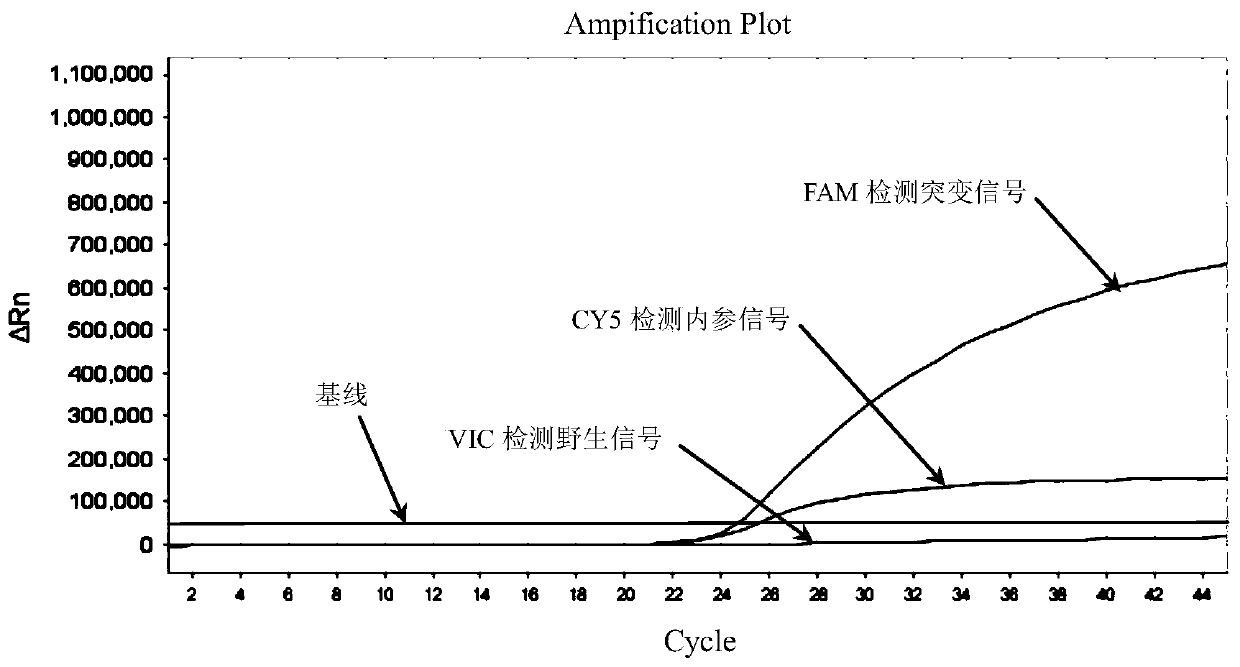
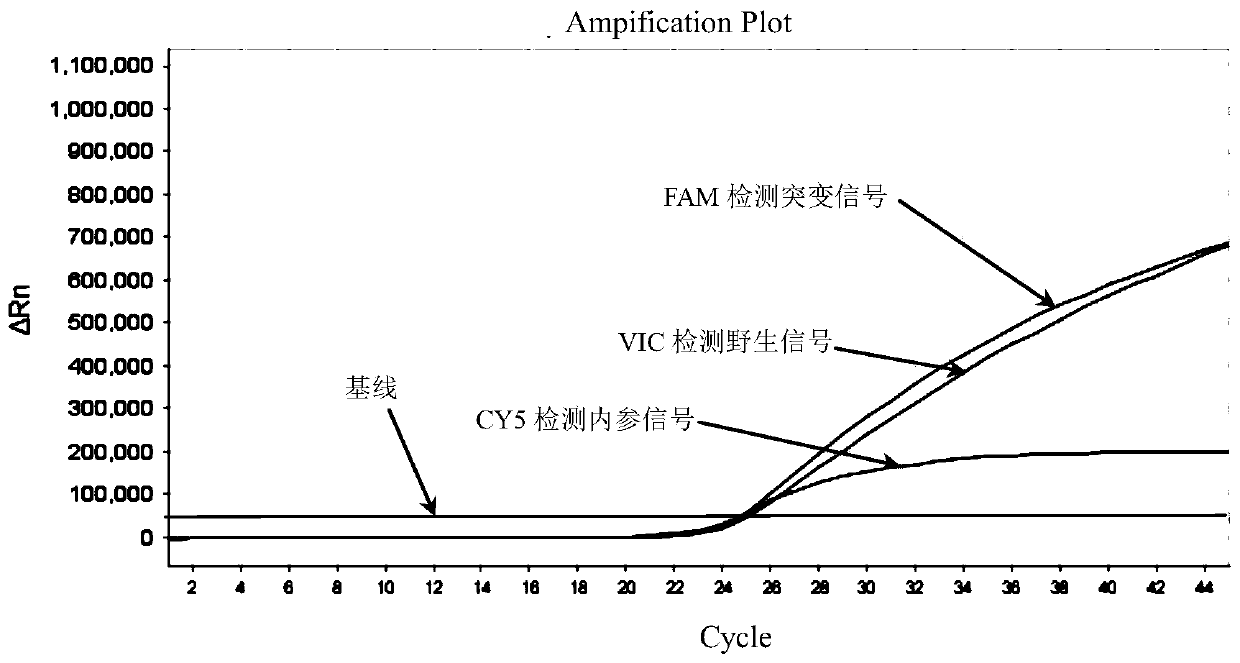
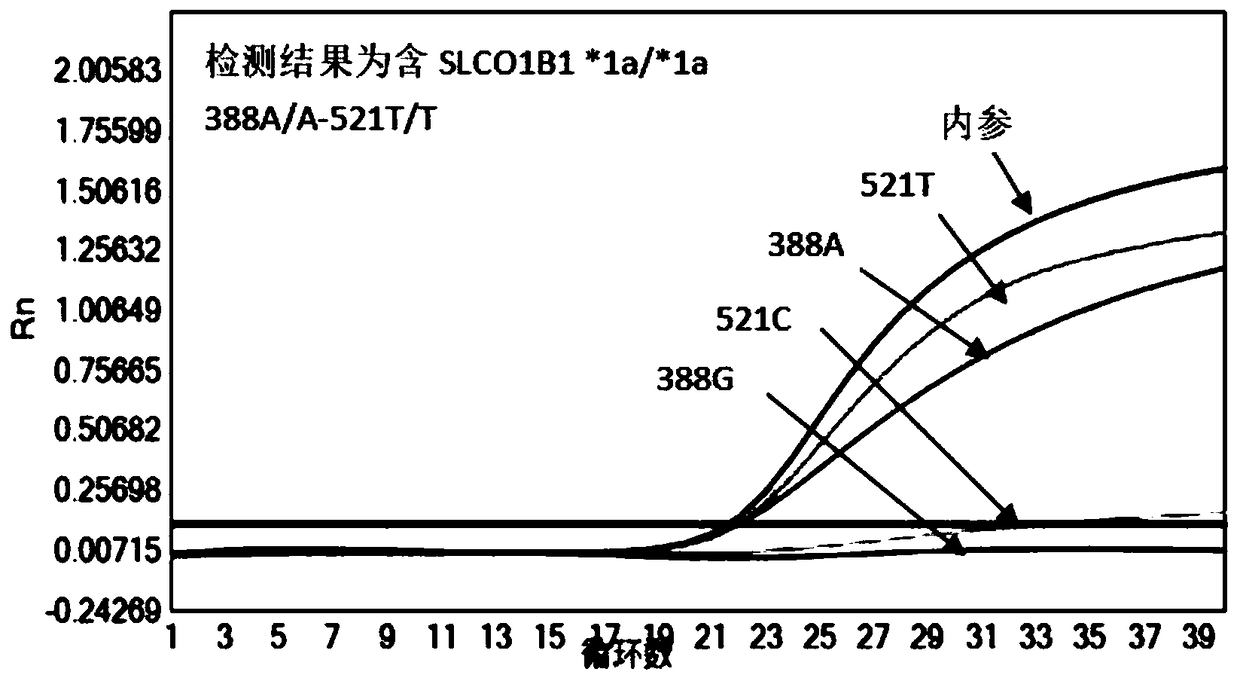
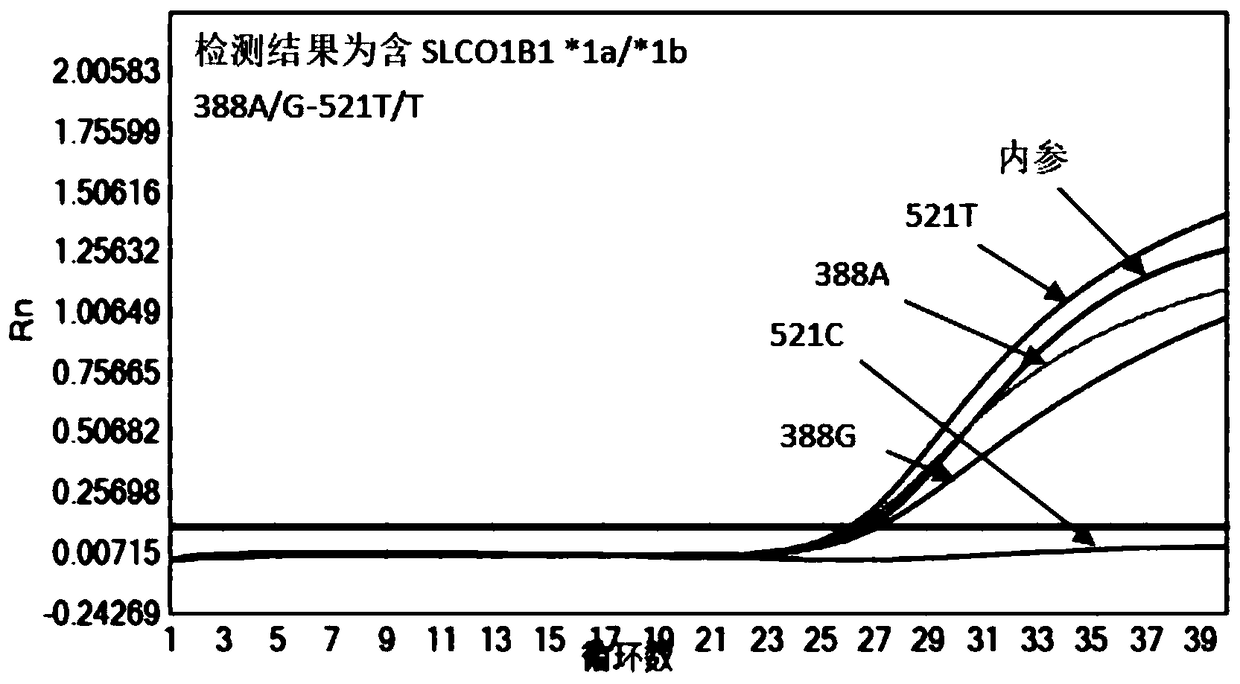
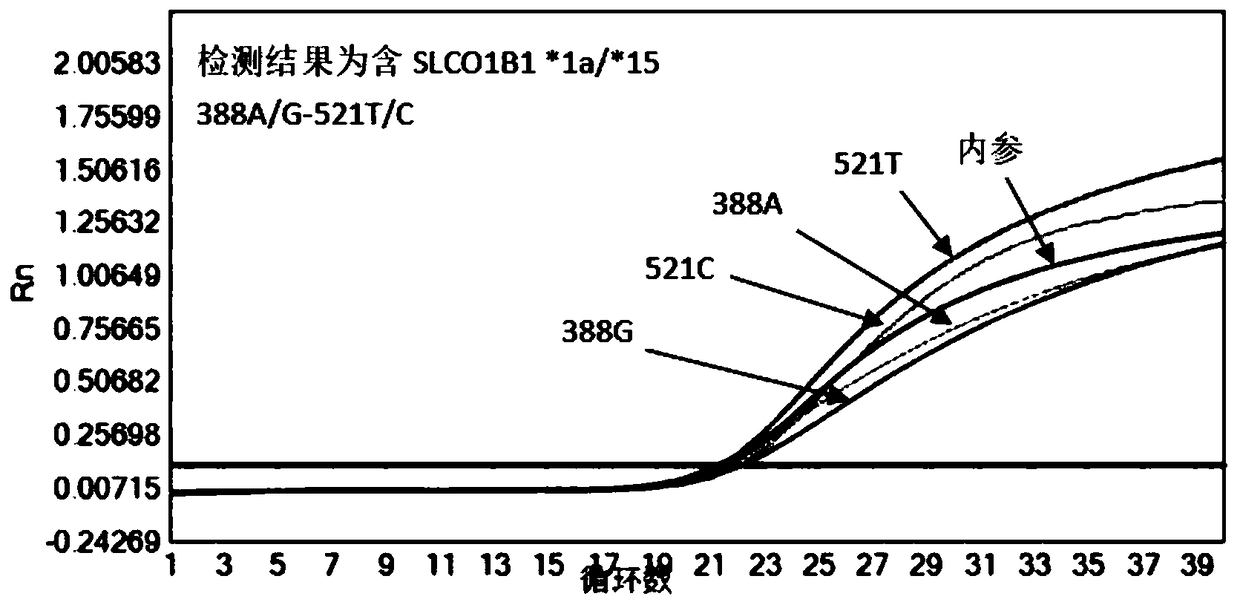
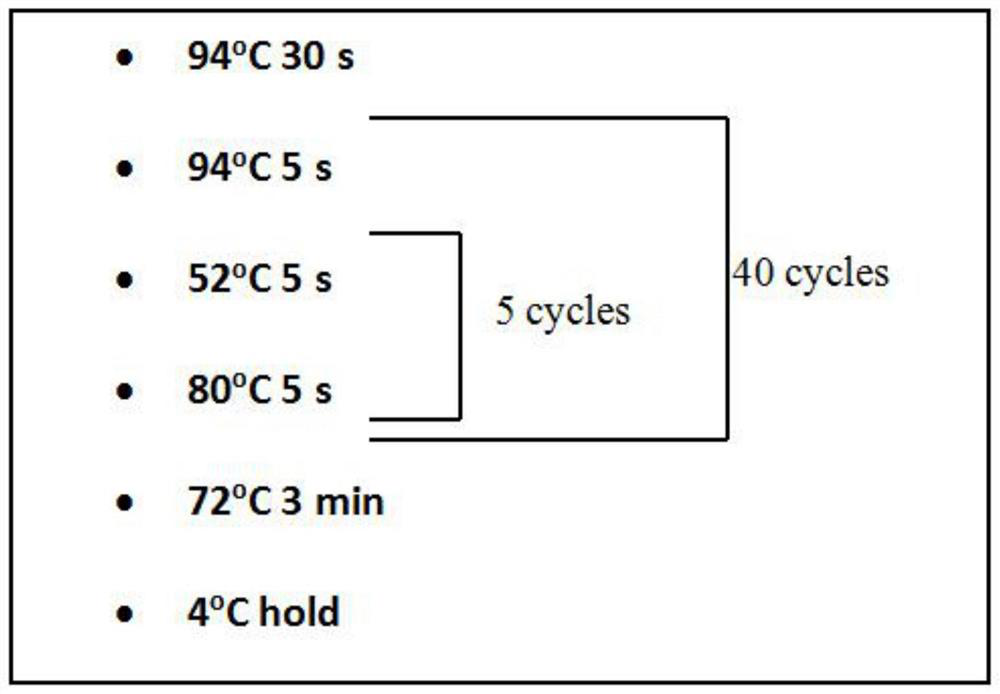
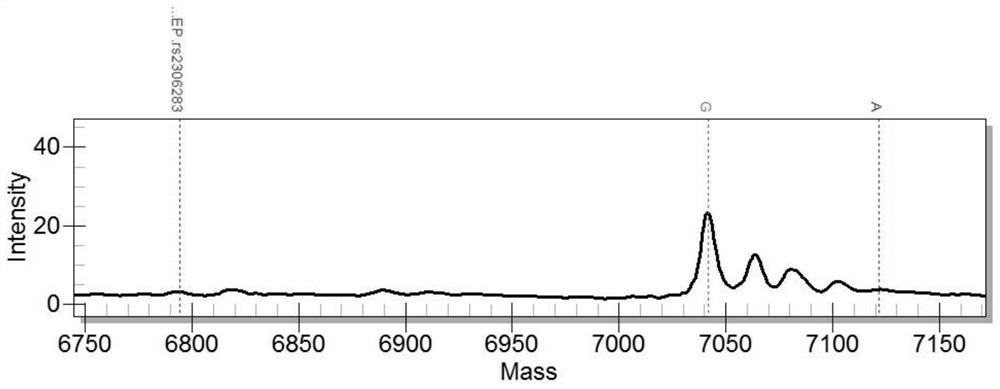
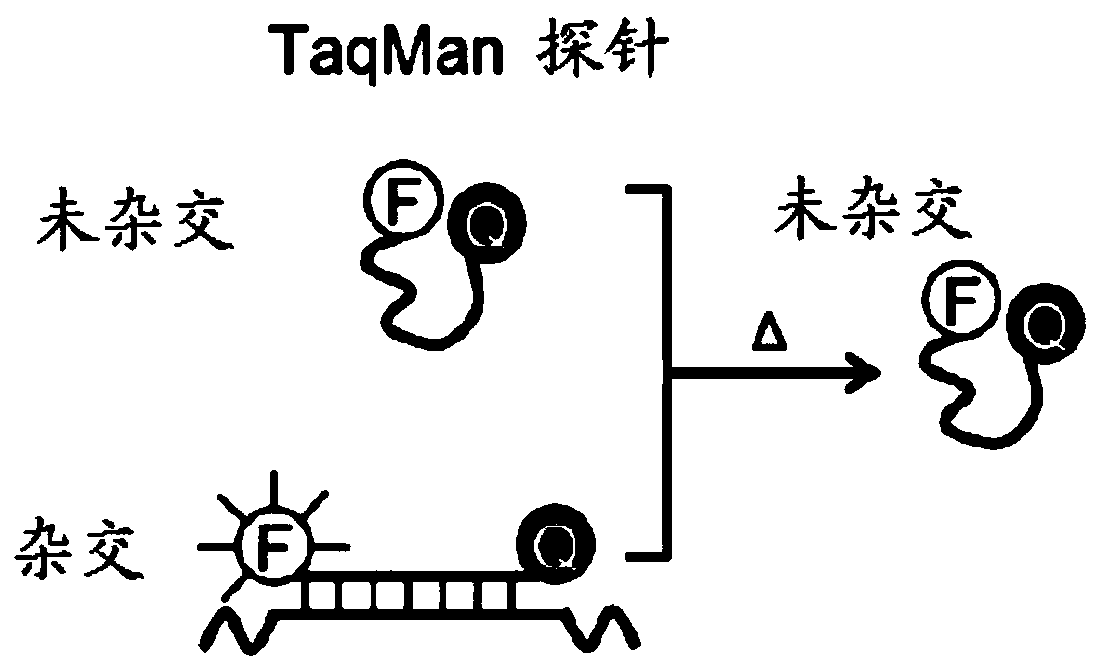
Patents
Literature
50 results about "SLCO1B1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solute carrier organic anion transporter family member 1B1 is a protein that in humans is encoded by the SLCO1B1 gene. Pharmacogenomic research indicates that genetic variations in this gene are associated with response to simvastatin. Clinical guidelines exist that can guide dosing of simvastatin based on SLCO1B1 gene variant using genotyping or whole exome sequencing.
Kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation
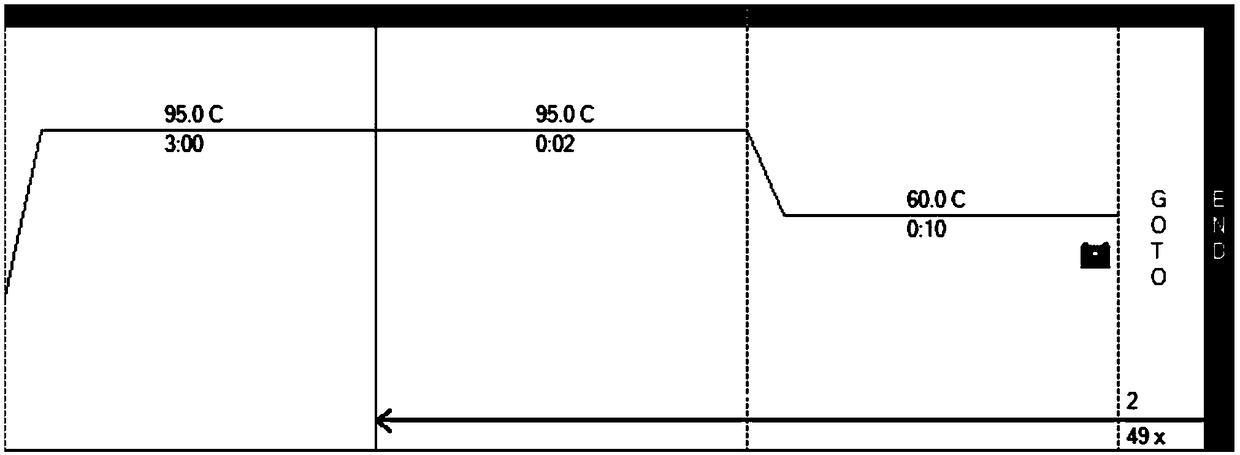
The invention belongs to the technical field of gene mutation detection, and concretely discloses a kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation. Through meticulous design, multi-time verification, screening and optimization, specific primers and probes based on a Taqman allelic gene resolution analysis method are obtained; eight functional variation of the SLCO1B1, APOE and LDLR genes can be detected; the time from DNA (Deoxyribonucleic Acid) extraction to fluorescent PCR (Polymerase Chain Reaction) to result obtaining is less than four hours; and the manual operation time is less than two hours. The kit comprising the primer pairs and the probe pairs has the advantages that the time is saved; convenience is realized; the sensitivity is high; and both the positive conformity rate and the negative conformity rate of a sample are higher than 99 percent, and the like. A detection method provided by the invention is mainly used for the personalized medication auxiliary diagnosis of statins such as simvastatin, atorvastatin, fluvastatin and rosuvastatin.
Owner:钟诗龙
Human SLCO1B1 and ApoE (apolipoprotein E) gene polymorphism detection kit
InactiveCN104846085AEasy to detectAccurate Typing DetectionMicrobiological testing/measurementApolipoprotein e4SLCO1B1
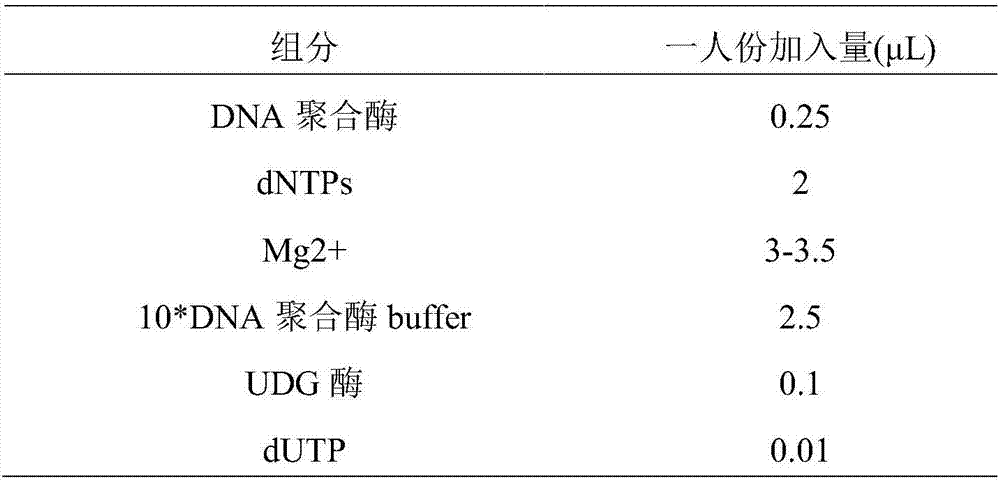
The invention provides a human SLCO1B1 and ApoE (apolipoprotein E) gene polymorphism detection kit, comprising a PCR (polymerase chain reaction) buffer solution, dNTP (deoxy-ribonucleoside triphosphate), MgCl2, four groups of specific primers, four groups of specific probes, an internal standard system, HotStart Taq enzyme and UNG (uracil-N-glycosylase) enzyme. The detection kit has the advantages of high specificity, high sensitivity, ease and quickness of operation, high throughput, safety, objectiveness of result interpretation, and the like.
Owner:WUHAN YZY MEDICAL SCI & TECH
A kit for simultaneously detecting statin metabolizing gene multisite mutations
The invention relates to a kit for simultaneously detecting statin metabolizing gene multisite mutations. The kit includes primer pairs and probe pairs for detecting APOE, SLCO1B1, CETP, ABCB1 and MTHFR gene sites. MGB (minor groove binder) probes and a real-time fluorescent PCR technique are applied in the kit. The kit has advantages of capability of being time saving and convenient, high sensitivity, capability of allowing positive and negative coincidence rates of a sample to be 99.5% or above, and the like. The kit is mainly used for personalized medication assisted diagnosis of statins such as simvastatin, atorvastatin and pravastatin.
Owner:NINGBO MEIJING MEDICAL TECH
Diagnostic methods
InactiveUS20110112186A1Altered protein expression levelEffective indicatorBiocideNervous disorderSLCO1B1Statine
This invention relates to a method of determining the susceptibility of an individual to statin-induced myopathy, comprising detecting the presence or absence of one or more polymorphisms in the SLCO1B1 gene in a biological sample from an individual, whereby the presence of one or more polymorphisms indicates that the individual has altered susceptibility to statin-induced myopathy.
Owner:ISIS INNOVATION LTD
Primers, probes and kit for detecting polymorphisms of ApoE gene and SLCO1B1 gene
ActiveCN106544419AAccurate detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Polymorphism Detection
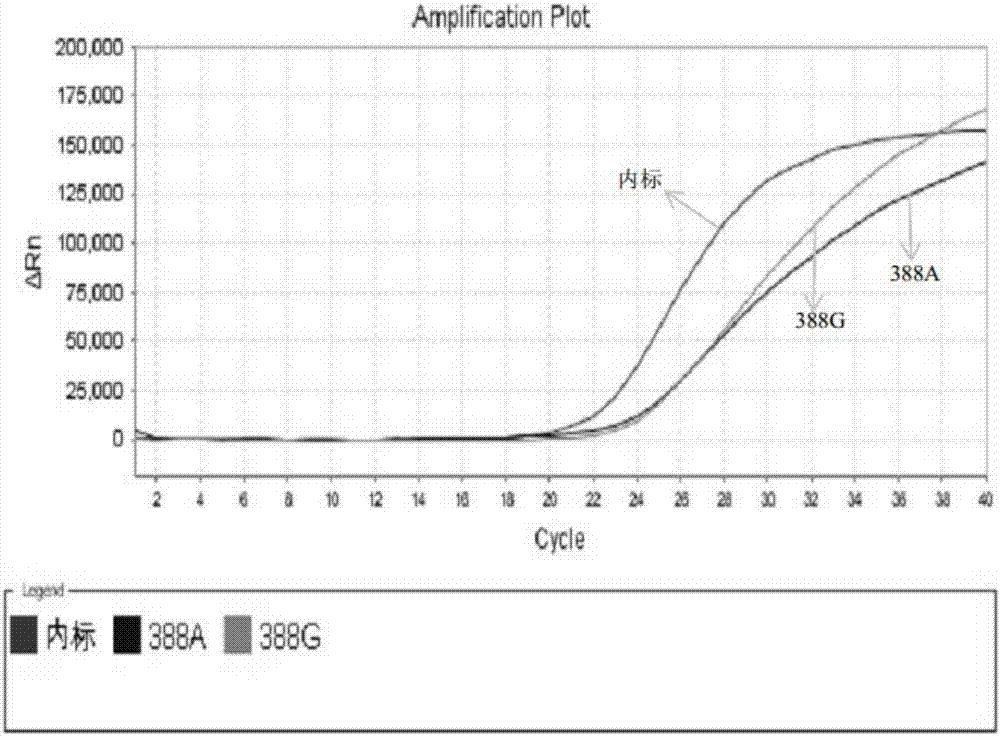
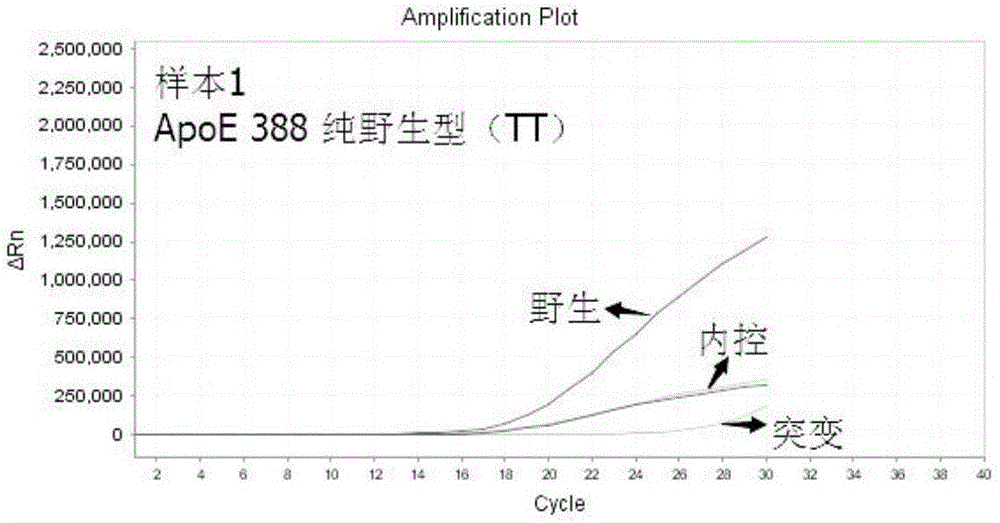
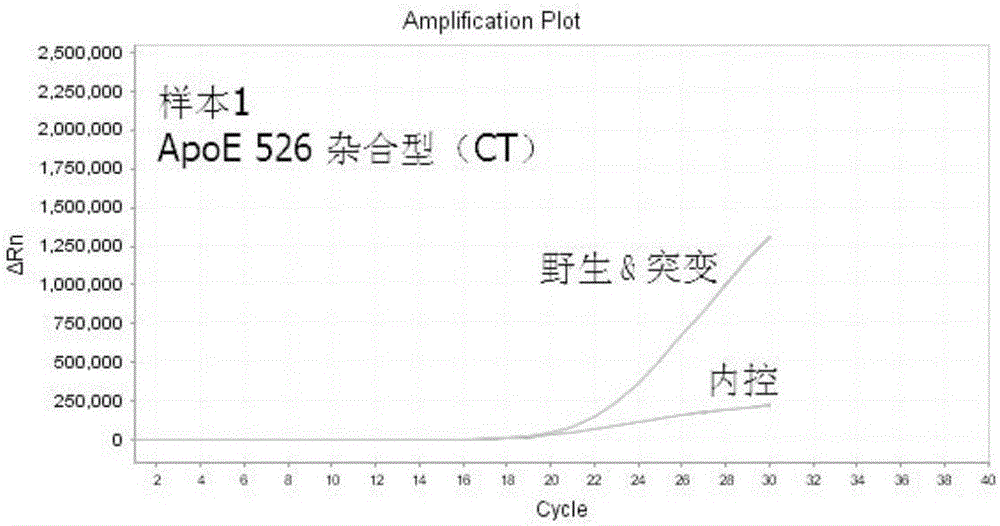
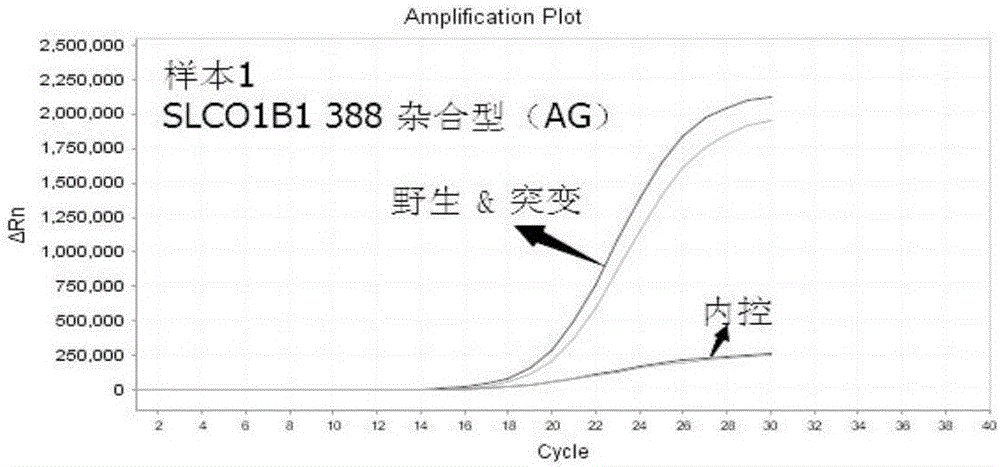
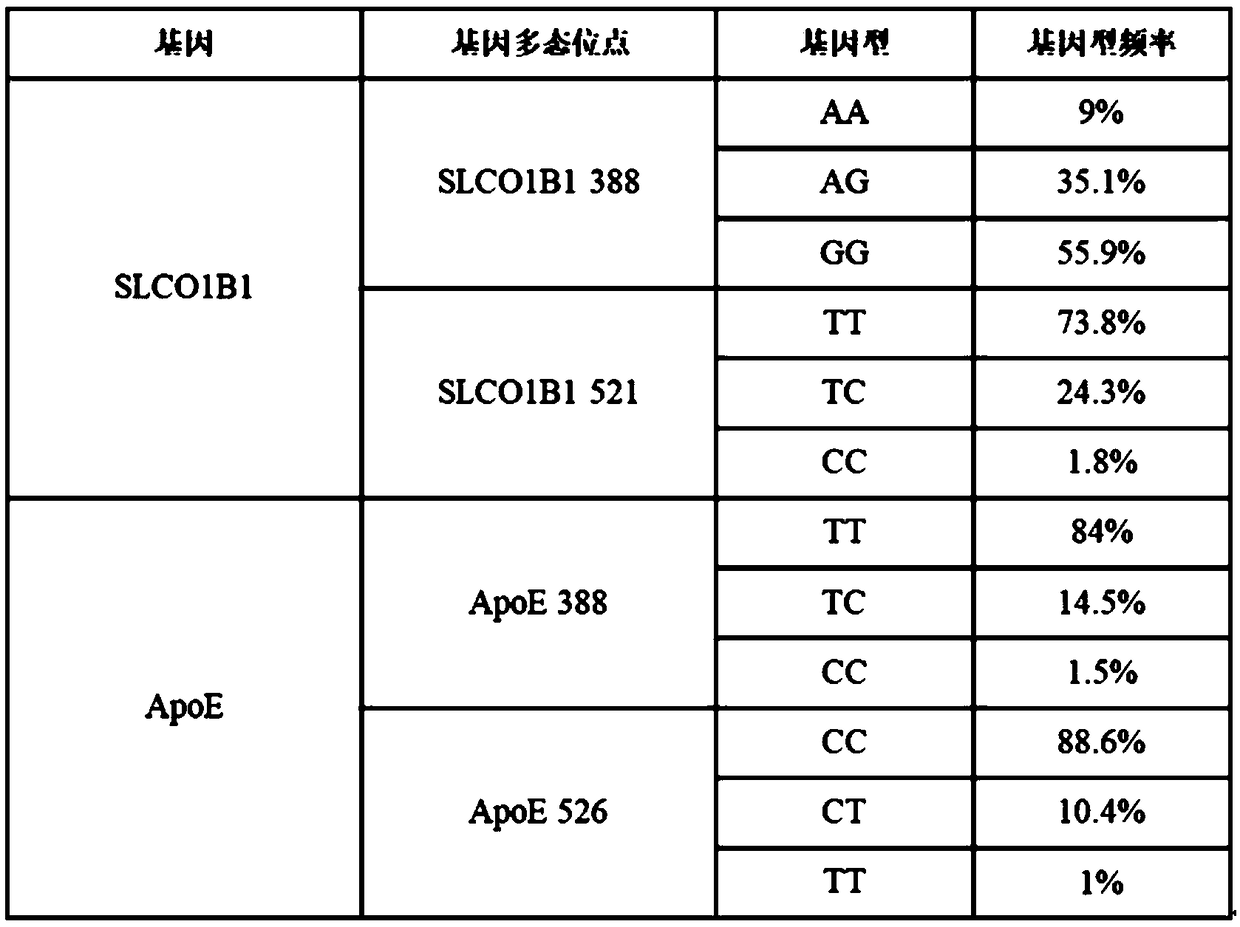
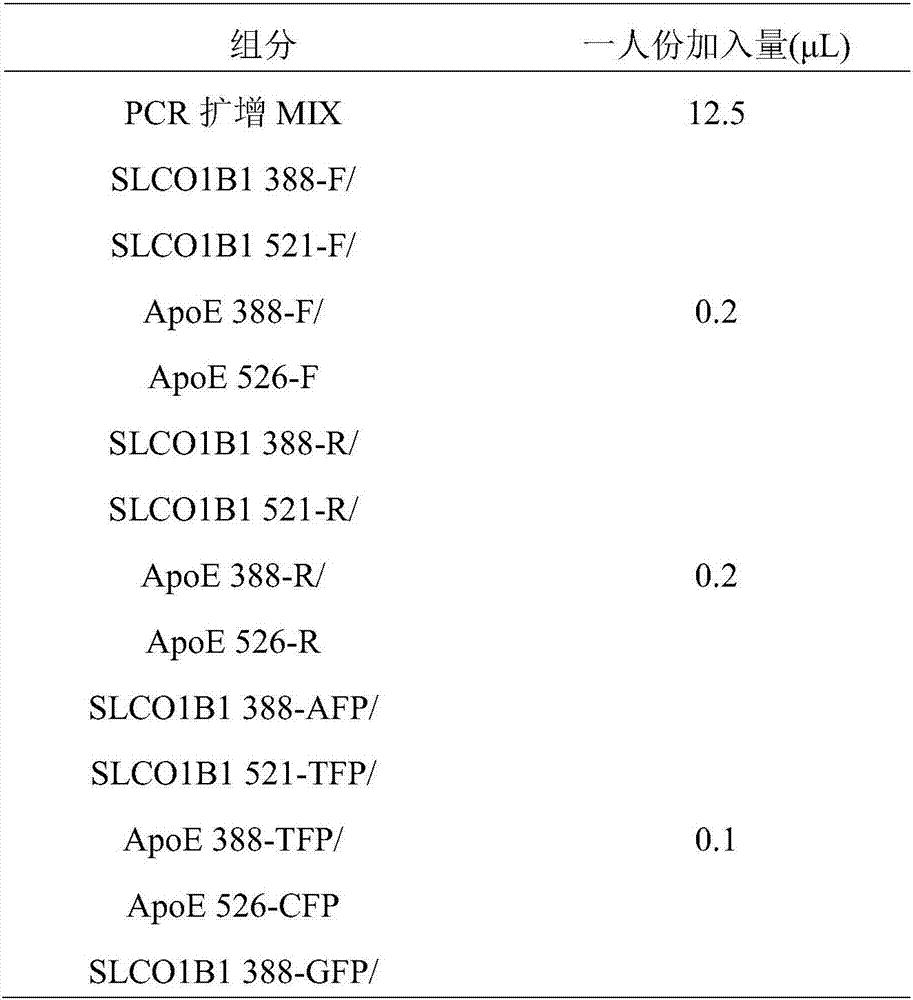
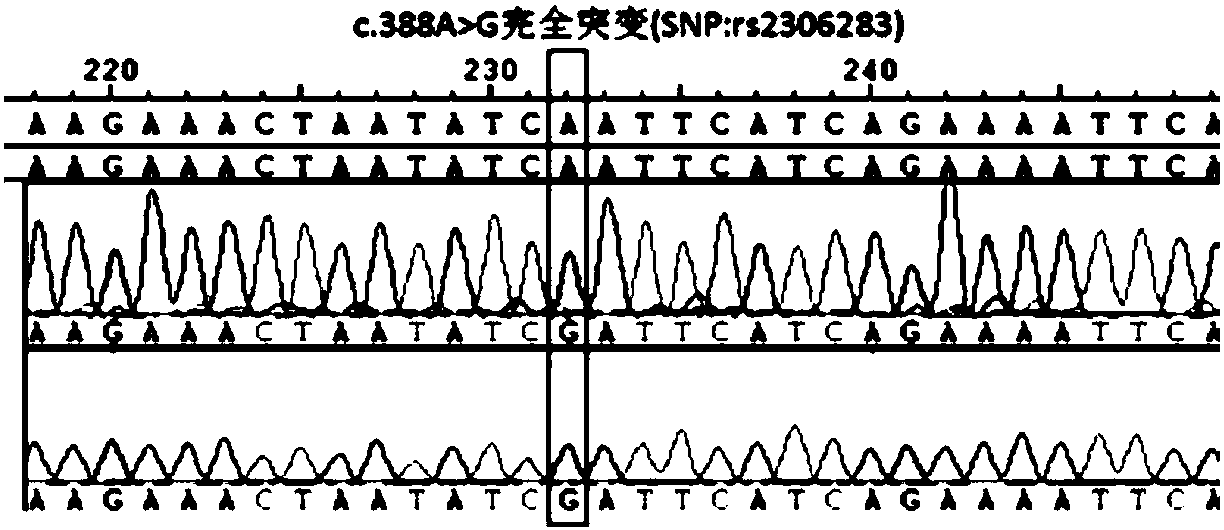
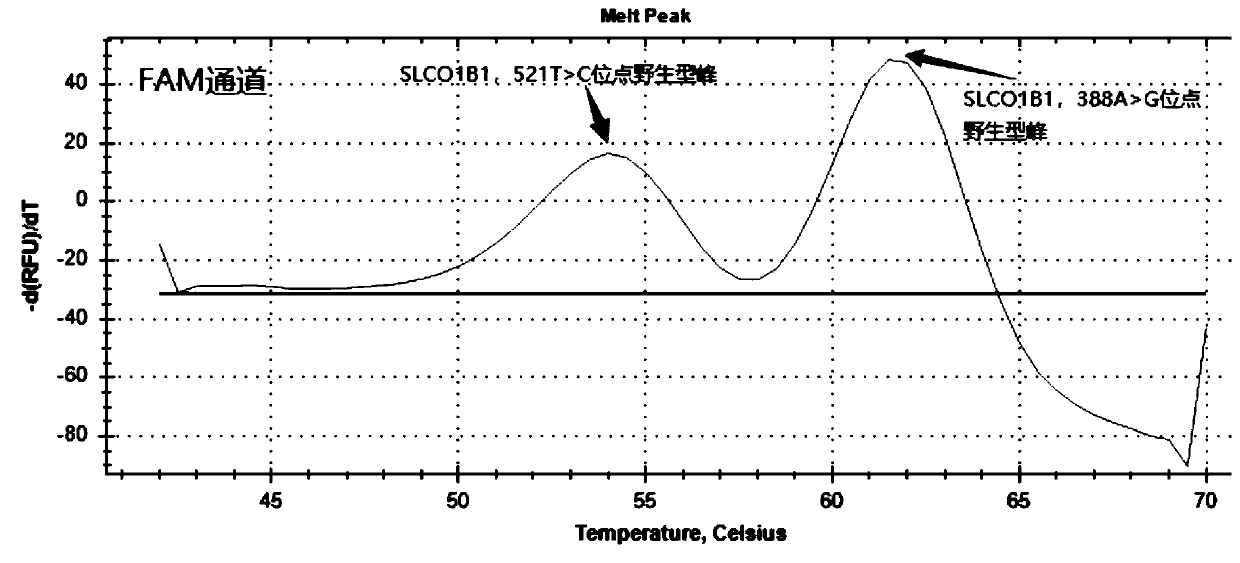
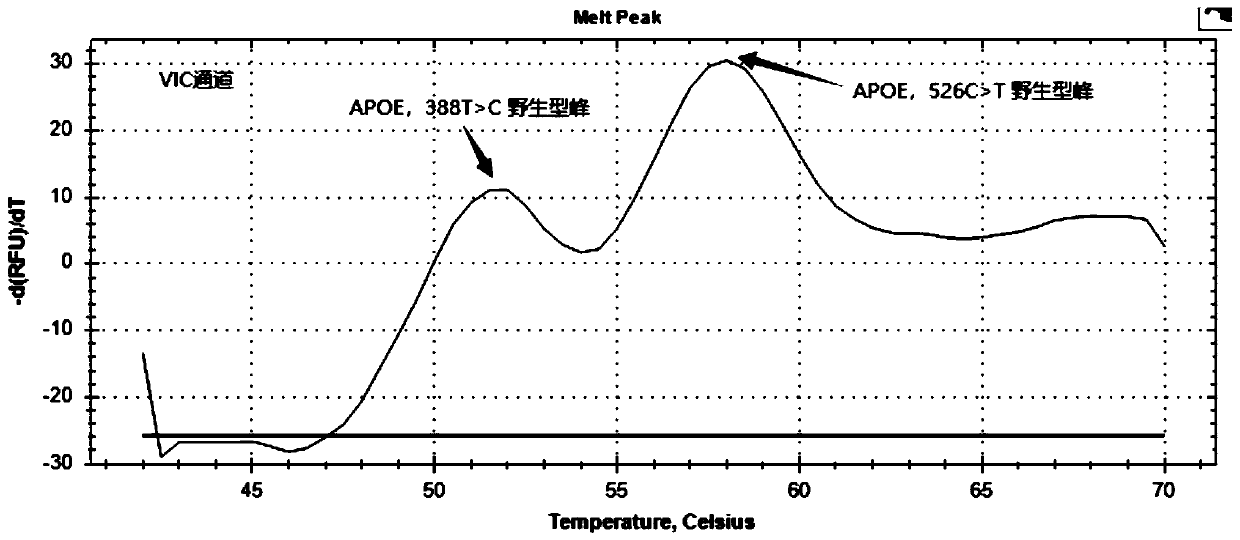
The present invention discloses primers, probes and a kit for detecting the polymorphisms of gene ApoE and gene SLCO1B1, wherein the primers and the probes comprise primers and probes for detecting the polymorphic loci of T388C and C526T of the gene ApoE, and primers and probes for detecting the polymorphic loci of A388G and T521C of the gene SLCO1B1. According to the present invention, the kit is suitable for the selection of a variety of clinical samples, and has significant advantages of high specificity, high sensitivity, short experimental period, simple operation, safety, no toxicity, low cost, and the like.
Owner:武汉海吉力生物科技有限公司
Human SLCO1B1 and ApoE gene polymorphism detection kit, and preparation method and application of same
ActiveCN108998517AGuaranteed accuracyReduce concentrationMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1True positive rate
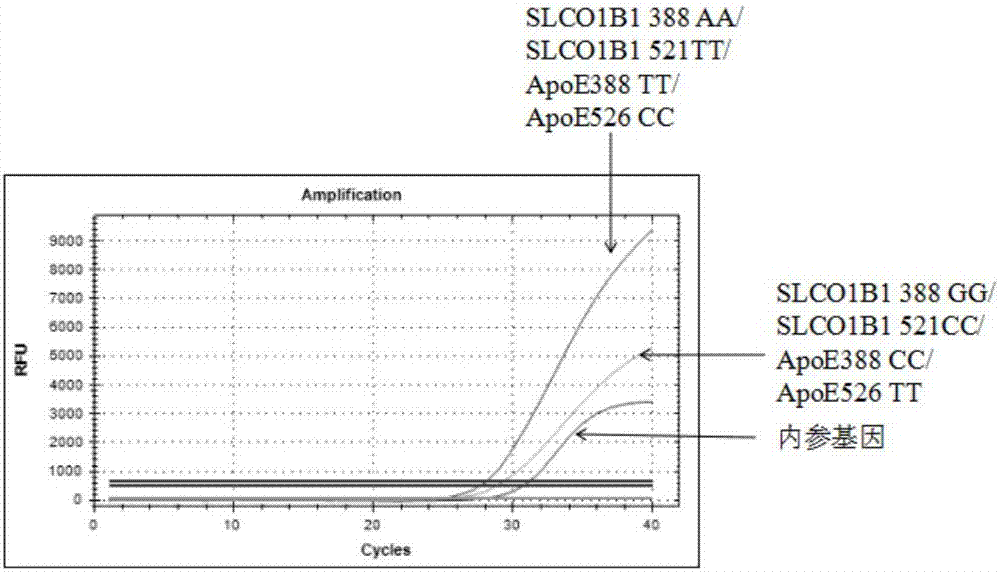
The invention belongs to the field of biotechnologies, and particularly relates to a human SLCO1B1 and ApoE gene polymorphism detection kit, and a preparation method and an application of same. The kit is composed of: a PCR premix reaction solution respectively used for detecting the rs2306283 loca of the SLCO1B1 gene, the rs4149056 loca of the SLCO1B1 gene, the rs429358 loca of the ApoE gene andthe rs7412 loca of the of the ApoE gene, and a positive reference substance and a negative reference substance. The PCR premix reaction solution includes specific primer sequence groups, probe groupsand a PCR reaction solution, which are used for amplifying the mentioned loci. The specific primer sequence groups are composed of a regular outer primer and specific ARMs primers having fluorescencetags. The kit is used for detecting the polymorphism of human SLCO1B1 and ApoE gene, is high in sensitivity and specificity, is easy to use and has reliable results, can complete the detection withinone hour, and is simple and objective in result interpretation.
Owner:WUHAN HEALTHCHART BIOLOGICAL TECH
Kit used for SNP detection of personalized medicine relaed genes of statin lipid-lowering medicine and detecting method thereof
InactiveCN106434940AHigh priceSolve efficiency problemsMicrobiological testing/measurementForward primerSide effect
The invention discloses a kit used for SNP detection of personalized medicine related genes of statin lipid-lowering medicine and a detecting method thereof. The kit comprises two forward primers and a reverse primer for detecting a locus rs17238540 of an the HMGCR gene, two forward primers and a reverse primer for detecting a locus rs7412 of an the APOE gene, two forward primers and a reverse primer for detecting a locus rs429358 of an the APOE gene, and two forward primers and a reverse primer for detecting a locus rs4149056 of an the SLCO1B1 gene. The kit used for the SNP detection of the personalized medicine related genes of the statin lipid-lowering medicine can conduct high throughput detection on the four loci, and a result of genotyping can be visually distinguished through software so as to achieve the purposes of quantitatively controlling metering of the statin lipid-lowering medicine and minimizing side effects.
Owner:中源协和基因科技有限公司
Composition for detecting gene polymorphism of SLCO1B1 and ApoE as well as application thereof
ActiveCN107541548ASimple and fast operationEasy to readMicrobiological testing/measurementDNA/RNA fragmentationProbe typeMultiplex pcrs
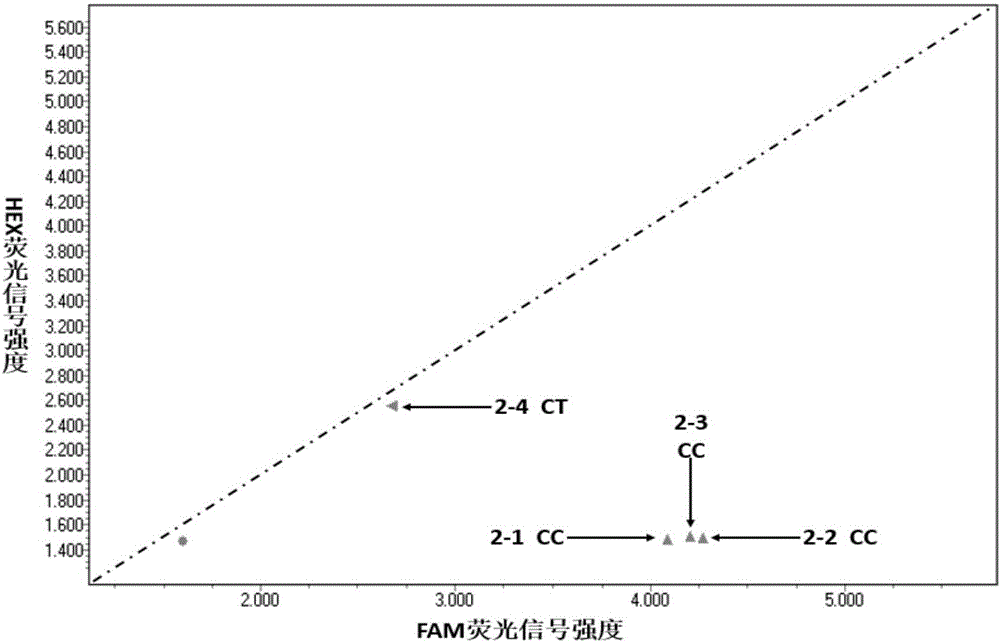
The invention discloses a composition for detecting gene polymorphism of SLCO1B1 and ApoE as well as an application thereof. An FAM, HEX, ROX (modified) three-channel multiple PCR reaction is used fordetecting single nucleotide polymorphism sites in SLCO1B1 and ApoE genes. In order to improve simplicity and specificity of detection, a probe typing technological means is used for realize one-tubetyping detection, and the whole operation and reaction process are simplified; on the basis, locked nucleic acid modification and secondary structure modification are carried out for the probe, Tm value of the product is added, in order to further improve specificity of probe combination; and finally proportioning of primer pairs, amount of Taq enzyme, amount of magnesium ions and the like are adjusted, in order to improve lowest detection limit, accuracy and specificity of the whole kit, lowest detection concentration of the kit reaches 1ng / [mu]L, and accuracy and specificity can reach 100%.
Owner:北京鑫诺美迪基因检测技术有限公司
Methods and compositions relating to pharmacogenetics of different gene variants in the context of irinotecan-based therapies
InactiveUS20090247475A1Reduced cytotoxic activityIncrease and reduce chance of responseBiocideMicrobiological testing/measurementPharmacogeneticsSLCO1B1
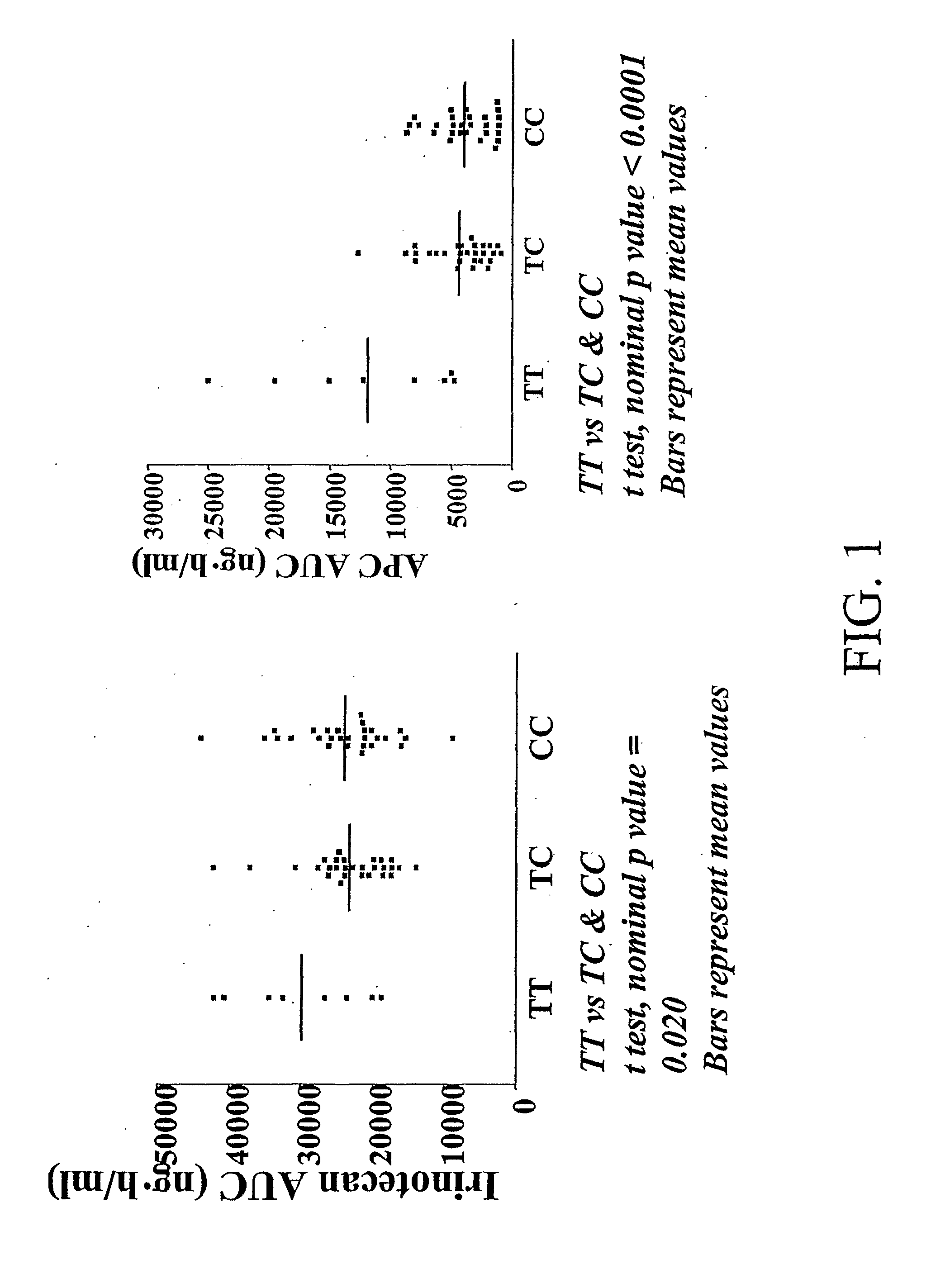
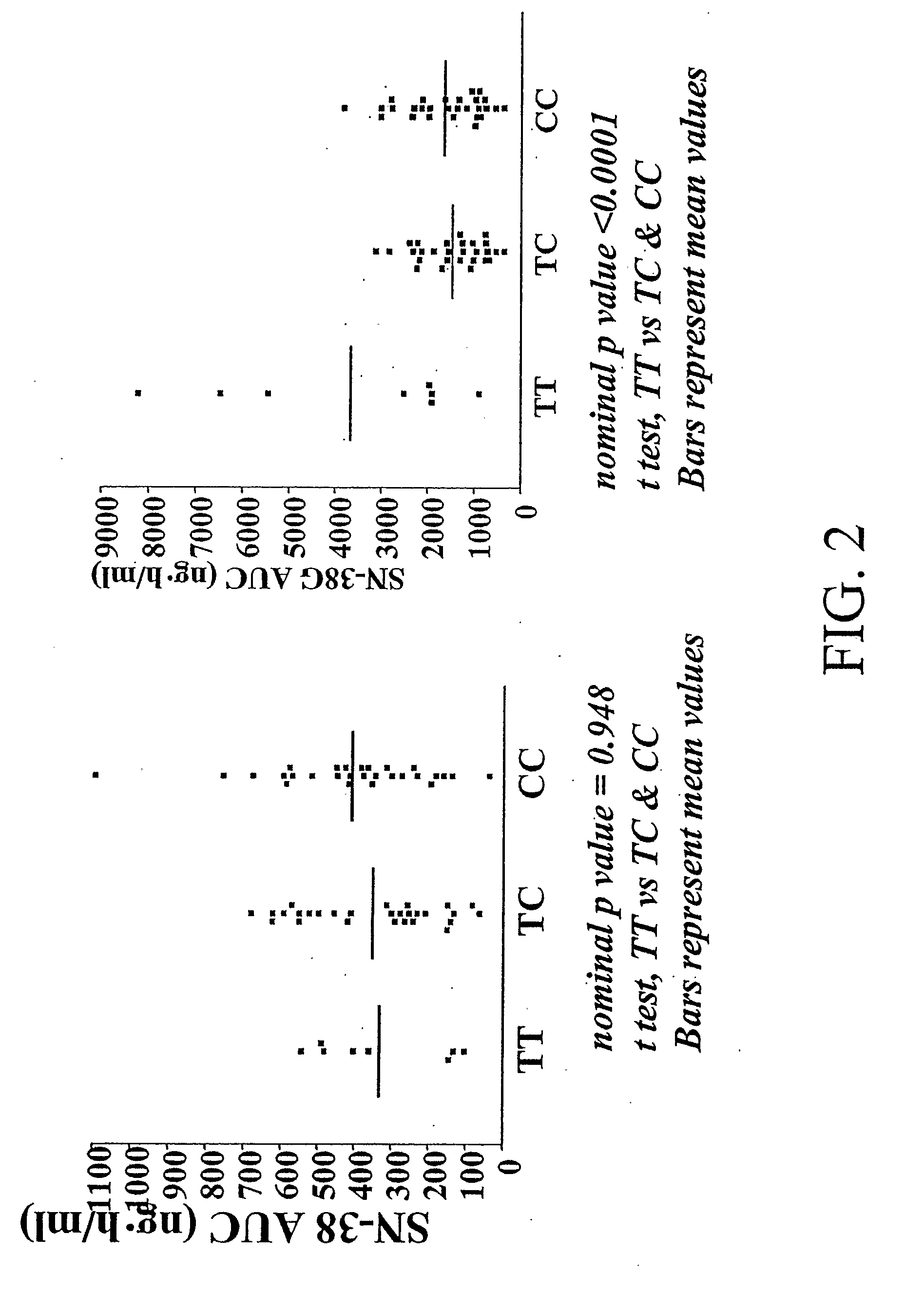
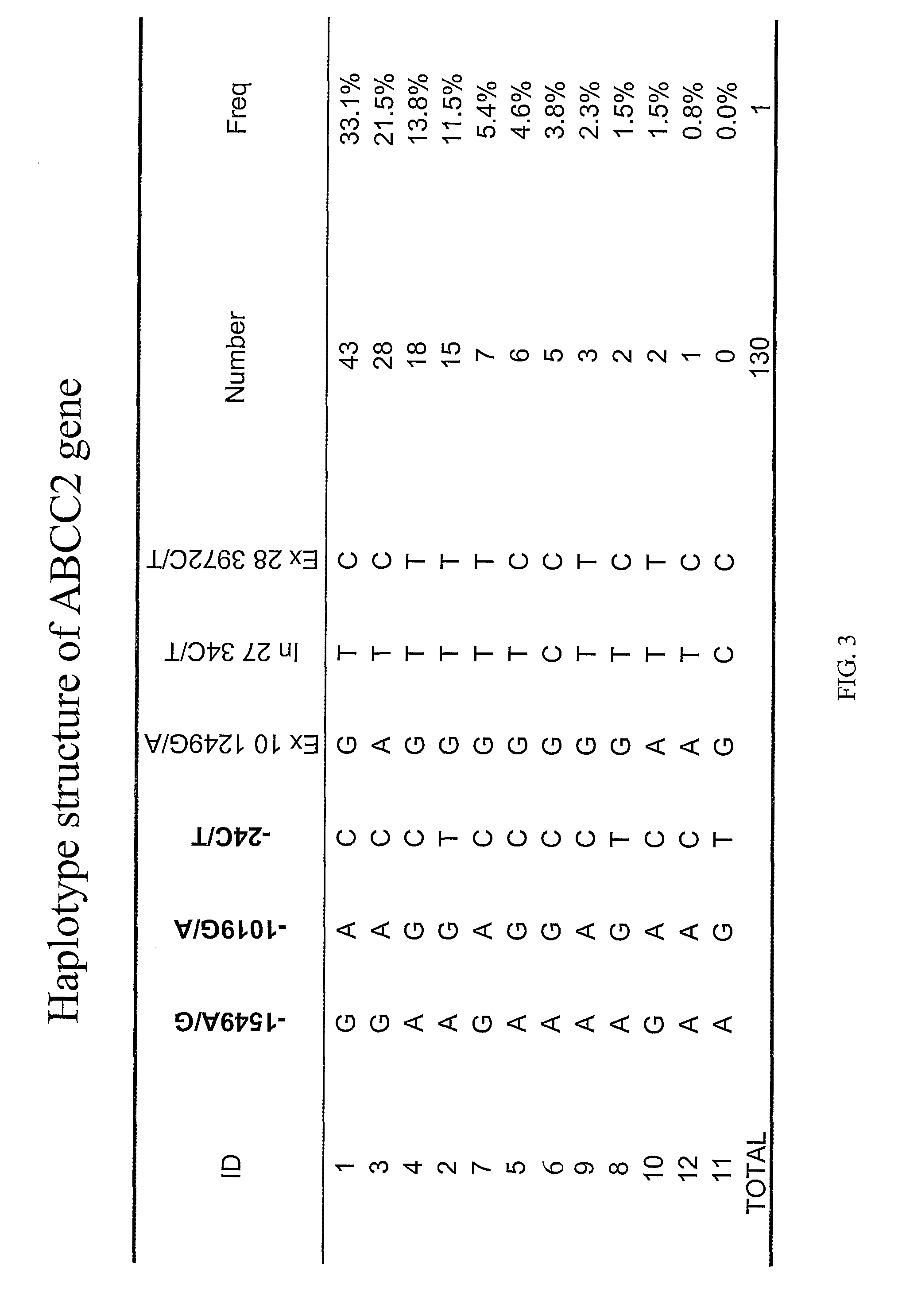
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:UNIVERSITY OF CHICAGO +1
Kit for detecting polymorphism of APOE gene and SLCO1B1 gene
InactiveCN108753951AEasy to operateAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationGeneticsFluorescent pcr
The invention provides a kit for detecting polymorphism of an APOE gene and an SLCO1B1 gene. The kit comprises primers used for detecting polymorphism sites T388C and C526T of the APOE gene and expressed as SEQ ID NO. 1-6, primers used for detecting polymorphism sites A388G and T521C of the SLCO1B1 gene and expressed as SEQ ID NO. 7-12, and internal quality control housekeeping gene primers expressed as SEQ ID NO. 13-20. The kit is used for carrying out fluorescence PCR by using ARMS-PCR and fluorescence fusion curve techniques, is capable of achieving accurate detection of one polymorphism oftwo genes in the same reaction tube, is high in sensitivity and high in specificity, and can meet detection of oral epithelial cells, dried-blood spots and whole-blood samples; the whole detection process is short in time; the medication basis can be provided for the doctor in the first time; the medication risks of the patients can be reduced.
Owner:SHENZHEN YOU SHENGKANG BIOSCI CO LTD
Primer-probe composition for detecting human SLCO1B1 and ApoE genetic typing, kit and detecting method
InactiveCN110387407ALow costHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Genetic typing
The invention provides a primer-probe composition for detecting human SLCO1B1 and ApoE genetic typing, a kit and a detecting method, the primer-probe composition comprises a specific primer, a specific probe and an internal reference agent for ApoE and SLCO1B1 genetic typing. According to the primer-probe composition, the kit and the detecting method, investigation of polymorphism of four types ofgenetic typing of SLCO1B1*1b(388G-521T), SLCO1B1*5(388A-521C), ApoE-E4(388C-526C) and ApoE-E2(388T-526T) in SLCO1B1 and ApoE genetic typing in a human body blood DNA sample is conducted synchronously, the advantages of high sensitivity, high specificity, low cost and high throughput, easy and convenient operation, and short experimental period are achieved, and the technical superiority of low cost of gene detecting is achieved.
Owner:WUXI SHENRUI BIO PHARMA
Kit and method for simultaneously and rapidly detecting SLCO1B1 and ApoE gene polymorphism
InactiveCN109457025AEasy to operateReduce operation timeMicrobiological testing/measurementSLCO1B1Whole blood sample
The invention discloses a kit and a method for simultaneously and rapidly detecting SLCO1B1 and ApoE gene polymorphism, and belongs to the technical field of gene detection. The kit comprises a sampletreating reagent, a gene testing reagent, a positive reference substance and a negative reference substance, the sample treating reagent is used for rapidly treating a human whole blood sample, and the gene testing reagent is separately packaged in a premixed single portion manner and includes an SLCO1B1 gene testing reagent and an ApoE gene testing reagent. According to the kit, convenient, rapid and efficient detection is achieved based on guarantee of detection specificity and sensitivity, detection steps are decreased, production cost and detection cost are reduced when detection reactiontime is shortened, and clinical detection, analysis and application are facilitated.
Owner:江苏美因康生物科技有限公司
Hyperlipemia susceptible noninvasive detection kit (APOB and other genes)
The invention provides a noninvasive detection kit for detecting hyperlipemia susceptible genes. The kit comprises specific primers and DNA (deoxyribonucleic acid) sequencing primers for detecting 3 mononucleotide polymorphism site genotypes (EcoRI on APOB gene, C677T (rs1801133) on MTHFR gene, and T521C (rs4149032) on SLCO1B1 gene), a PCR (polymerase chain reaction) component, a PCR product purification component, a DNA sequencing reaction component and the like. The kit evaluates the hyperlipemia attack risk level by detecting the 3 mononucleotide polymorphism site genotypes closely related to hyperlipemia on the gene level, and instructs people to prevent the occurrence of diseases in advance. The sampling method is an oral mucosa cell sampling method which is painless and noninvasive and avoids cross infection. The kit has the advantage of accurate and reliable sequencing detection result, and does not need to buy expensive imported special instruments; and thus, the method is easy to popularize.
Owner:解码(上海)生物医药科技有限公司
Establishment of methodology for detecting genes affecting efficacy of lipid-lowering drugs by TaqMan-MGB probe technique
InactiveCN109897896AEasy to readImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationDrug interactionSLCO1B1
The invention discloses a method for detecting genes affecting the efficacy of lipid-lowering drugs by combining a TaqMan-MGB probe and a real-time fluorescence quantitative PCR technique. The polymorphism of the genes affecting the efficacy of the lipid-lowering drugs in samples to be detected is detected, six SNP loci can be detected simultaneously, used primers and the TaqMan-MGB probe have high specificity, the risk of false positive caused by PCR product contamination can be reduced, the operation is simple and the result is accurate and reliable. According to the method, functional meaningful mutations such as SLCO1B1 c.521T>C (rs4149056), SLCO1B1 c.388A>G(rs2302683), SLCO1B1 c.-910G>A(rs4149015), ApoE c.388T> C(rs429358), ApoE c.526C>T(rs7412) and NPC1L1 c.-18C>A(rs41279633) are detected to assist clinicians to select appropriate drugs and doses or avoid occurring of drug interactions, the best therapeutic effect for patients is achieved, and thus the purpose of real ''individualized medication'' is achieved.
Owner:江苏百世诺医疗科技有限公司
Method of Predicting Increased Risk of Suffering Statin-induced Adverse Drug Reactions
InactiveUS20140005281A1Increased riskBiocideMicrobiological testing/measurementNachr subunitSlow channel syndrome
Inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (statins) are prescribed to lower serum cholesterol levels and reduce the risk of CVD. Despite the success of statins, many patients abandon treatment owing to neuromuscular adverse drug reactions (ADRs). Genome-wide association studies have identified the single-nucleotide polymorphism (SNP) rs4149056 in the SLCO1B1 gene as being associated with an increased risk for statin-induced ADRs.By studying slow-channel syndrome transgenic mouse models, this invention determined that statins trigger ADRs in mice expressing the mutant allele of the rs137852808 SNP in the nicotinic acetylcholine receptor (nAChR) α-subunit gene CHRNA1. Mice expressing this allele show a remarkable contamination of end-plates with caveolin-1 and develop early signs of neuromuscular degeneration upon statin treatment. The invention demonstrates that genes coding for nAChR subunits may contain variants associated with statin-induced ADRs.
Owner:LASALDE JOSE A +4
Specific primmer and liquid phase chip for SLCO1B1 gene SNP detection
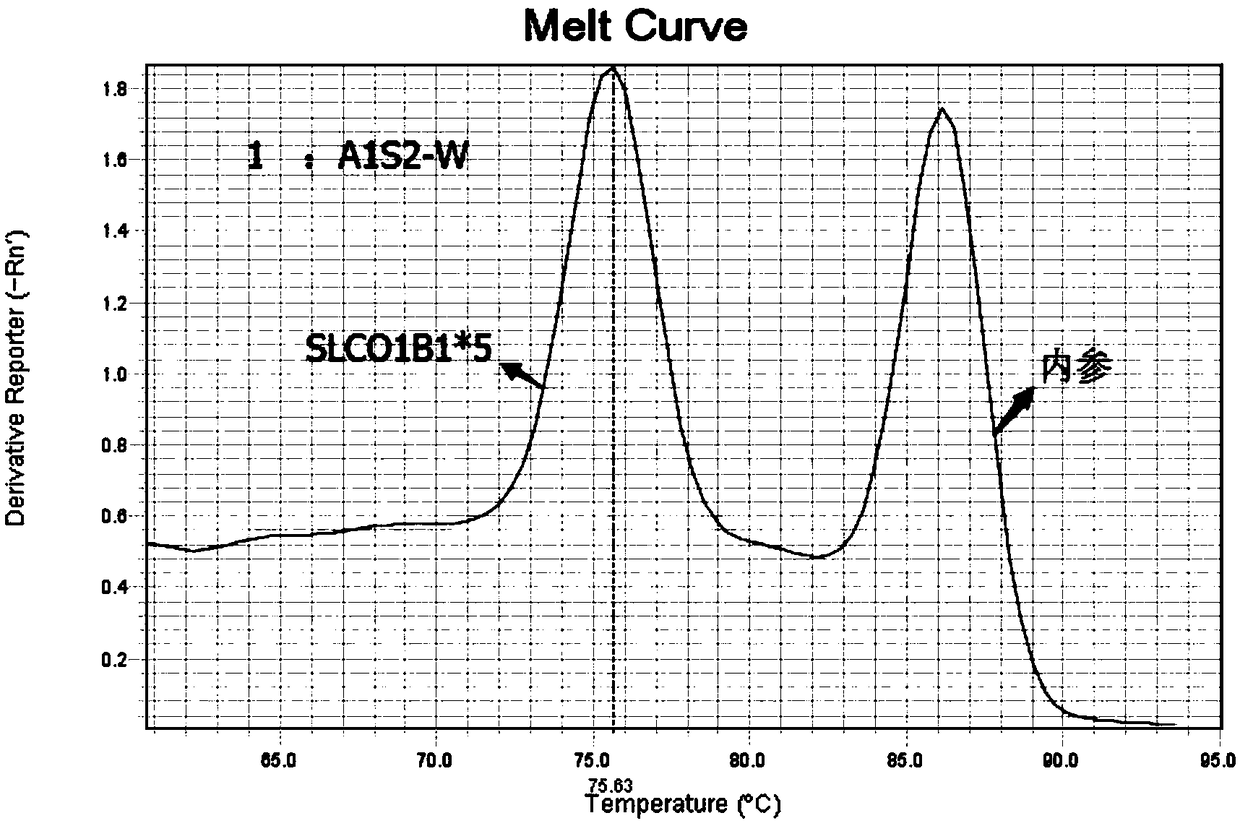
InactiveCN102021239AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationSignal-to-noise ratio (imaging)SLCO1B1
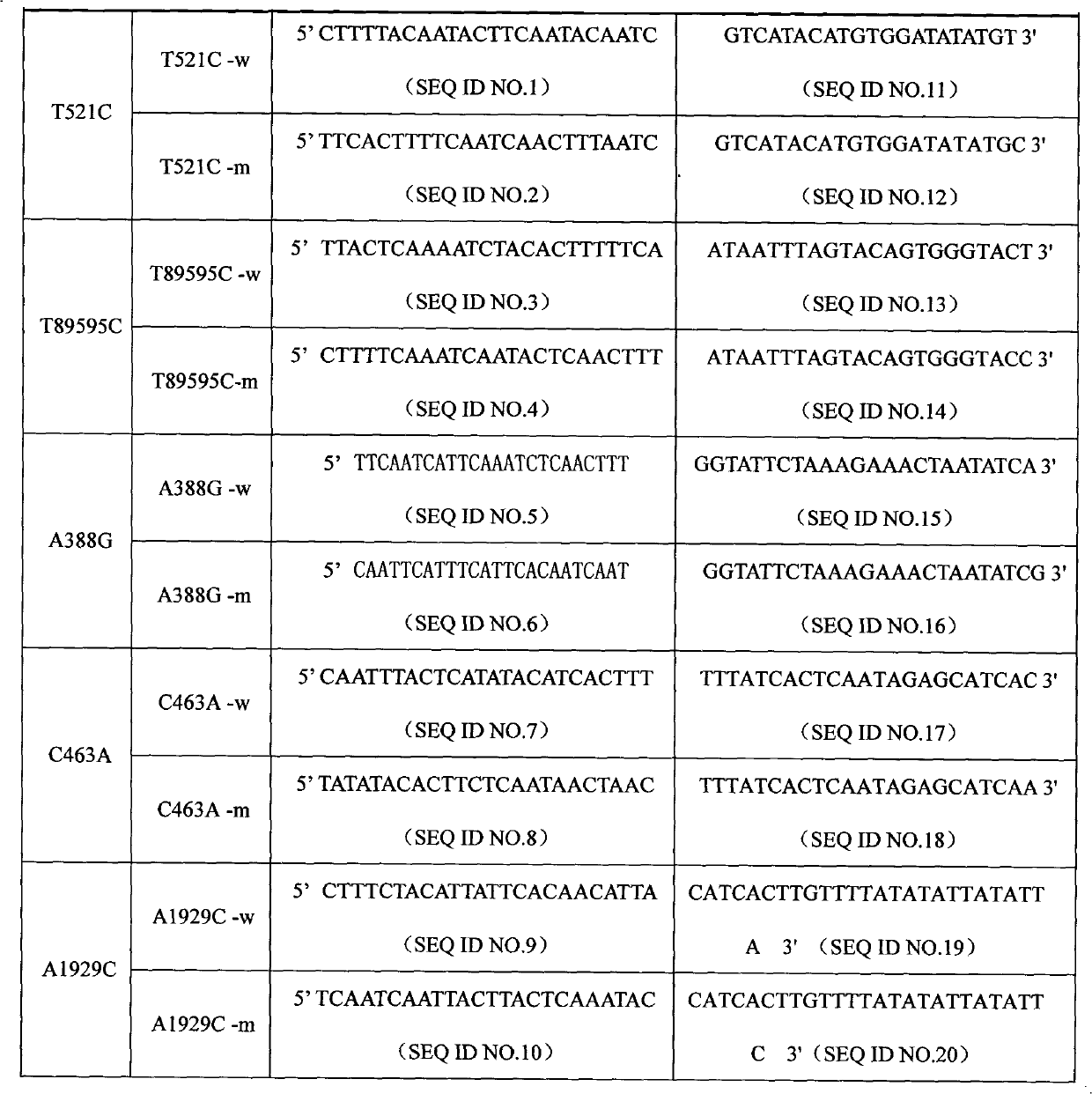
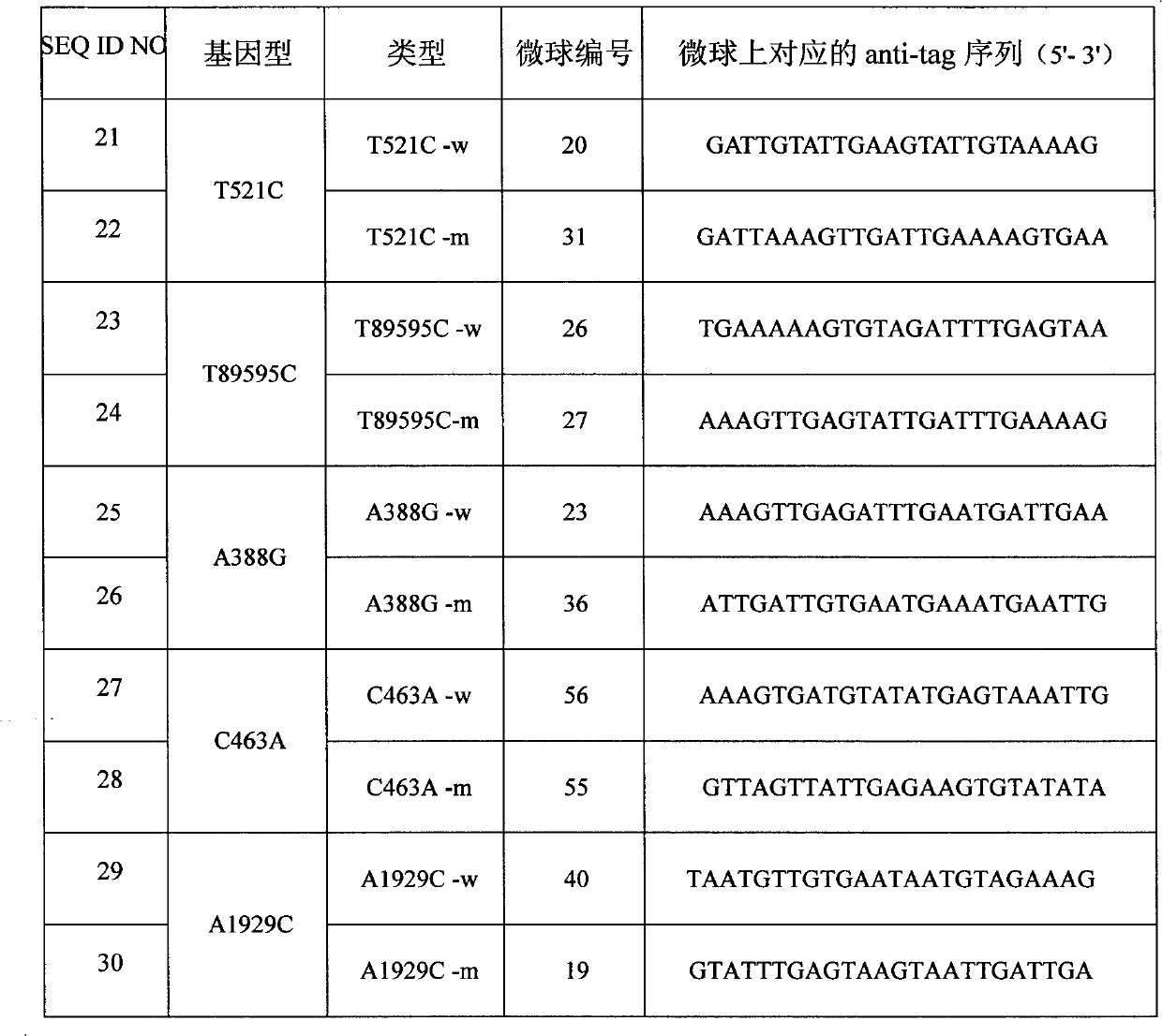
The invention discloses a specific primmer and a liquid phase chip for solute carrier organic anion transporter 1B1 (SLCO1B1) gene signal nucleotide polymorphism (SNP) detection. The liquid phase chip mainly comprises allele specific primer extension (ASPE) primer pairs, microspheres and amplifiers, wherein each ASPE primer consists of a tag sequence at a 5' end and specific primers at a 3' end aiming at an SNP locus; the specific primers comprise SEQ ID NO.11 and SEQ ID NO.12 aiming at T521C SNP locus, SEQ ID NO.13 and SEQ ID NO.14 aiming at T89595C SNP locus, SEQ ID NO.15 and SEQ ID NO.16 aiming at A388G SNP locus, SEQ ID NO.17 and SEQ ID NO.18 aiming at C463A SNP locus, and / or SEQ ID NO.19 and SEQ ID NO.20 aiming at A1929C SNP locus; and the tag sequence is selected from SEQ ID NO.1 to SEQ ID NO.10. The coincidence rate of the liquid phase chip provided by the invention and the detection result of a sequencing method reaches 100 percent. And the prepared liquid phase chip for the SLCO1B1 gene SNP detection has high signal-to-noise ratio.
Owner:SUREXAM BIO TECH
Primer combination for guiding related gene detection of individualized medications of risuvastatin drug, kit and method
InactiveCN110387412AReduce adverse reactionsEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Analysis method
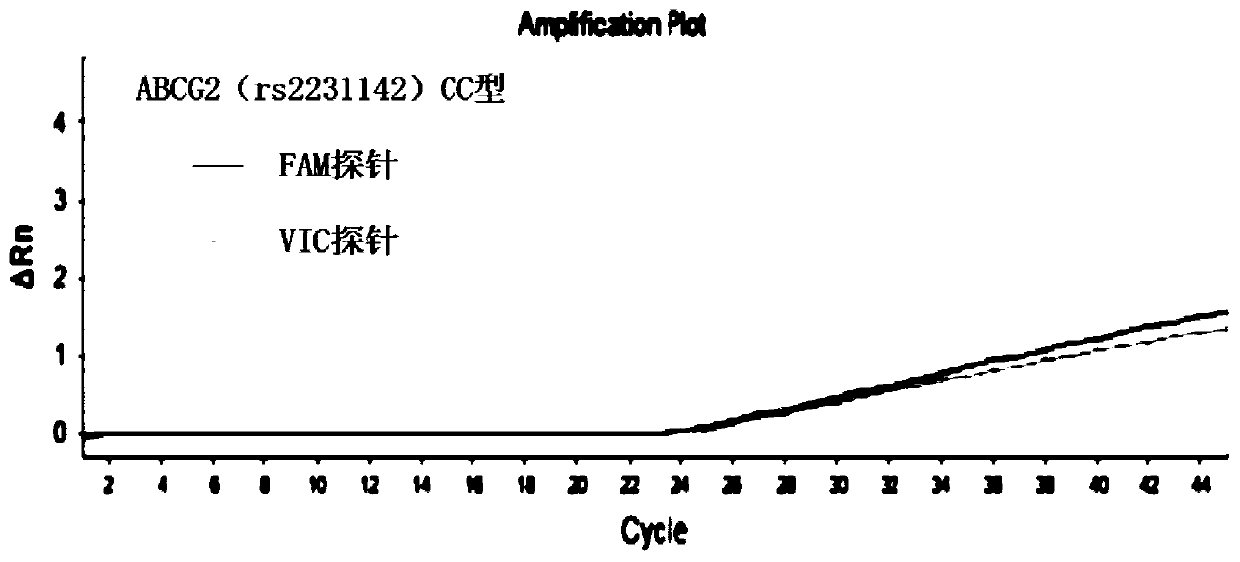
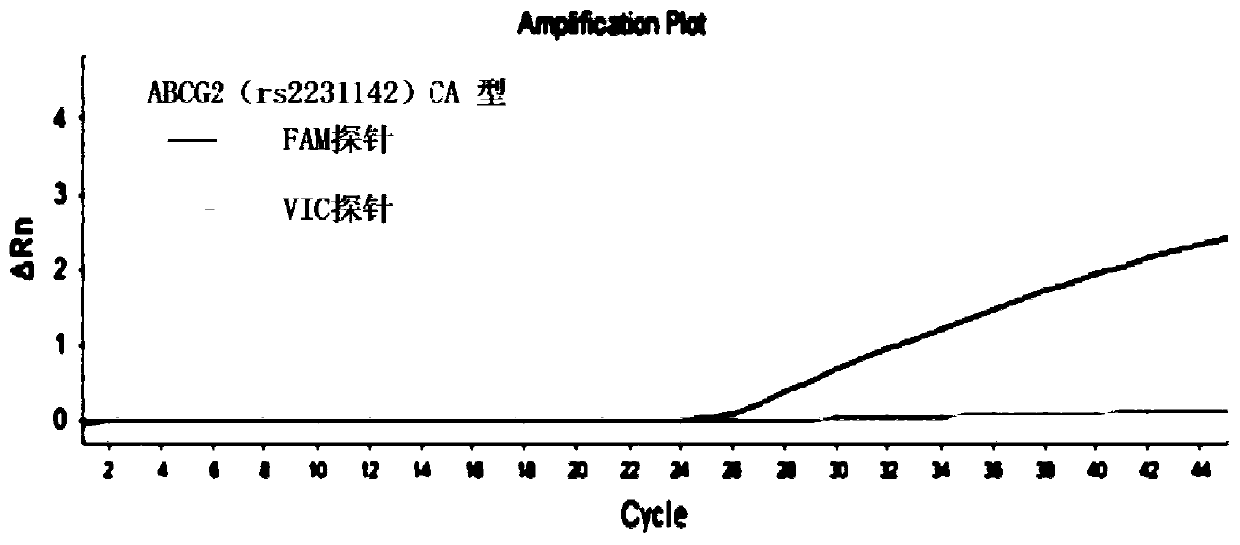
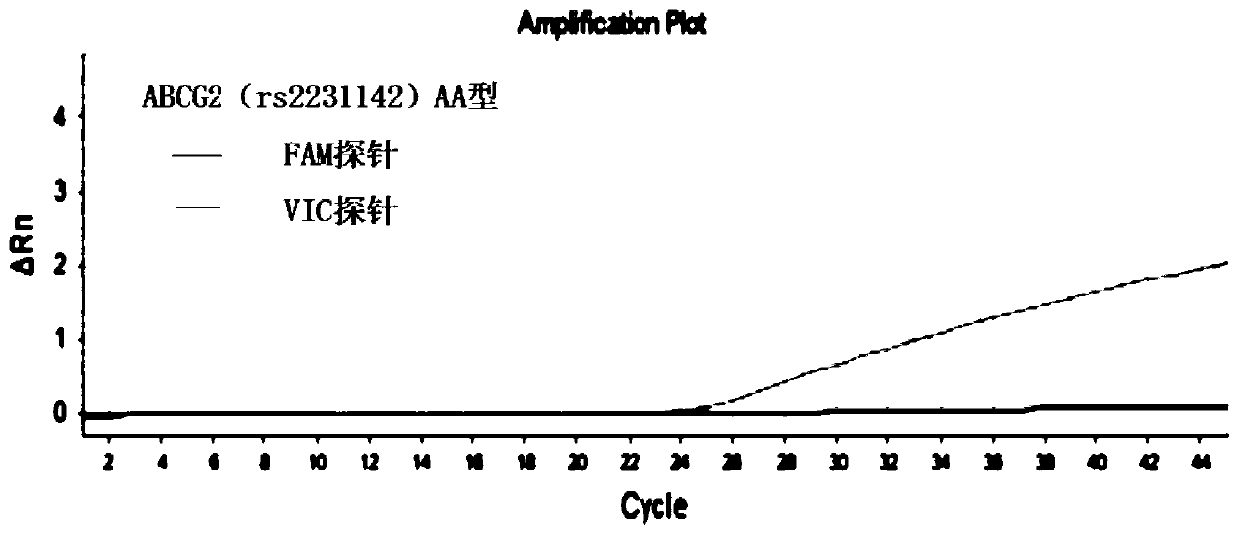
The invention discloses a primer combination for guiding related gene detection of individualized medications of a risuvastatin drug. The primer combination is characterized by including specific primers and probes for an ABCG2(rs2231142) gene locus and an SLCO1B1(rs4149056) gene locus. The invention further discloses a kit containing the primer combination and an analytical method of the kit. According to the primer combination, the characteristics of easy operation, the economical efficiency, rapid detection, the high accuracy, the good specificity and easy result interpretation are achieved, the polymorphism of related genes of the individualized medications of the risuvastatin drug is rapidly and accurately measured to lower the adverse reaction of common medications of the rosuvastatin drug in clinic, the medical cost is lowered, and social resources are saved.
Owner:SHANGHAI PASSION BIOTECHNOLOGY CO LTD
Kit for detecting human hyperlipidemia sensitive genes
InactiveCN112410415AAccurate detectionEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Medicine
The invention discloses a kit for detecting human hyperlipidemia sensitive genes. The kit comprises an APOE primer group, an SLCO1B1-1 primer group, an SLCO1B1-2 primer group, a COQ2 primer group anda CYP3A5 primer group. The human hyperlipidemia drug sensitive genes APOE, SLCO1B1, COQ2 and CYP3A5 are taken as detection objects. Through a combination of the specific primers and combination of a nucleic acid mass spectrometry detection technology, a rapid, simple and accurate detection of variation conditions of the related genes is realized.
Owner:为康(苏州)基因科技有限公司
Method, primer and kit for detecting SLCO1B1 gene mutation
InactiveCN108315397ASimple equipment requirementsEasy to judgeMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Individualized treatment
The invention discloses a primer, a method and a kit for detecting SLCO1B1 gene mutation related to CHD (Coronary Atherosclerotic Heart Disease). The primer comprises two pairs of amplification primers aiming at a fifth exon sequence and a sixth exon sequence and a pair of sequencing primers; by adopting a Sanger sequencing technology, gene mutation related to the CHD can be rapidly detected. A result of detection completed by utilizing the primer, the method and the kit, which are disclosed by the invention, is accurate, and an important reference significance in early diagnosis and individualized treatment of the CHD is obtained.
Owner:南昌艾迪康医学检验实验室有限公司
Compositions and methods for treating and preventing coronary heart disease
The invention pertains to a method of determining a statin dosage for an individual in need of treatment with a statin, comprising determining a SLCO1B1 genotype from a nucleic acid sample of the individual, said genotype comprising the presence or absence of the SLCO1B1-056 polymorphism, and determining an ApoE genotype or phenotype identifying an ApoE polymorphism selected from the group consisting of ApoE2, ApoE3, ApoE4, and any combination thereof, wherein the combination of a SLCO1B1 genotype identifying the presence of the SLCO1B1-056 C polymorphism and the ApoE genotype or phenotype identifying one of the ApoE3 / 4 or ApoE4 / 4 genotypes indicates the statin dosage.
Owner:BOSTON HEART DIAGNOSTICS
SNP fluorescence in situ hybridization sequencing detection method for ABCB1 and SLCO1B1
InactiveCN109295229AEasy to identifyAccurate readingMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceSLCO1B1
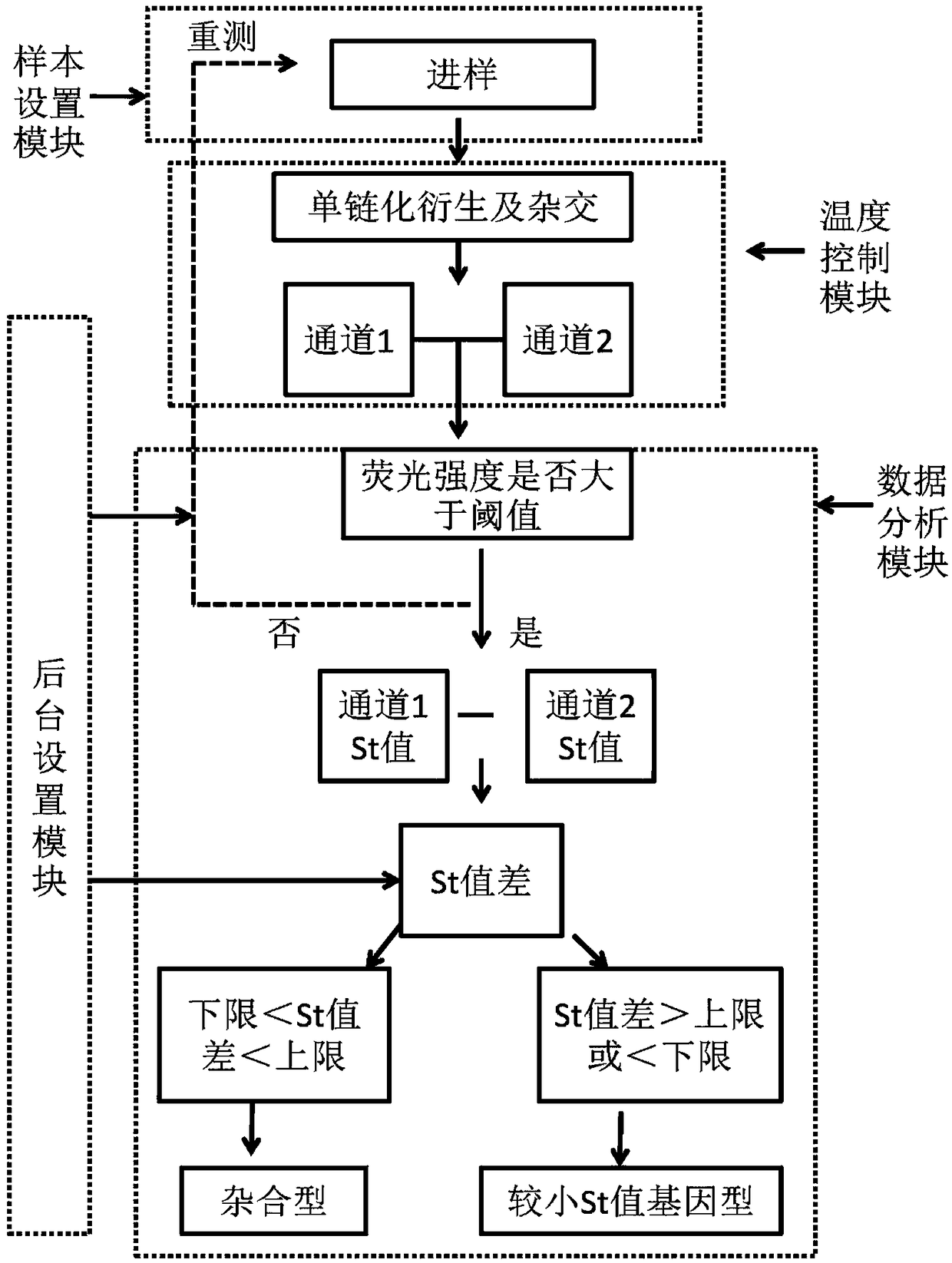
The invention discloses a SNP fluorescence in situ hybridization sequencing detection method for ABCB1 and SLCO1B1. The method comprises the following steps: DNA of a sample to be tested is extracted;single-stranded derivatization is performed using the DNA as a template; different fluorescently-labeled first sequencing probes and second sequencing probes are simultaneously added to be hybridizedwith the single-stranded derivative; and finally, the hybridization result is interpreted. The method has high precision and good stability, and is fast, safe and easy for automatic operation. By themethod, precise typing of single nucleotide polymorphism can be completed in the cycle of single-stranded derivatization and hybridization reaction, thereby understanding the genotype of the subjectand achieving prevention of diseases caused by risk factors and clinical guidance for individualized medication.
Owner:北京华夏时代生物工程有限公司
Detection kit for methotrexate metabolic markers and detection method and application of detection kit
PendingCN113528629AShorten the timeImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationDrug utilisationSLCO1B1
The invention discloses a detection kit of methotrexate metabolism markers, and a detection method and application of the detection kit. The kit is used for detecting the gene polymorphism of the methotrexate metabolism markers ABCB1 (C3435T), MTHFR (C677T) and SLCO1B1 (c.1865+4846T>C). According to the invention, multiple RPA amplification and optimized pyrosequencing technologies are combined to detect the methotrexate drug dosage and the gene polymorphism related to adverse reaction prediction, the kit can be used for simultaneously detecting ABCB1 (C3435T), MTHFR (C677T) and SLCO1B1 (c.1865+4846T>C) gene polymorphism methotrexate drugs, and suggestions from the gene perspective are provided for clinical personalized medication.
Owner:湖南菲思特精准医疗科技有限公司
Primer combination used for guiding lovastatin drug individualized medication related gene detection and kit and method
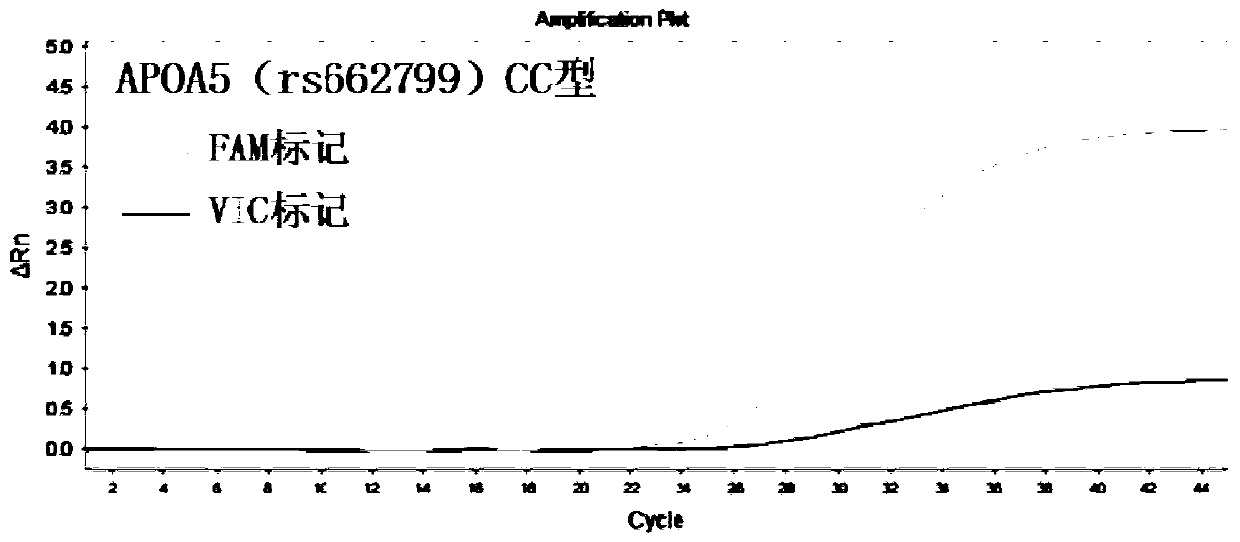
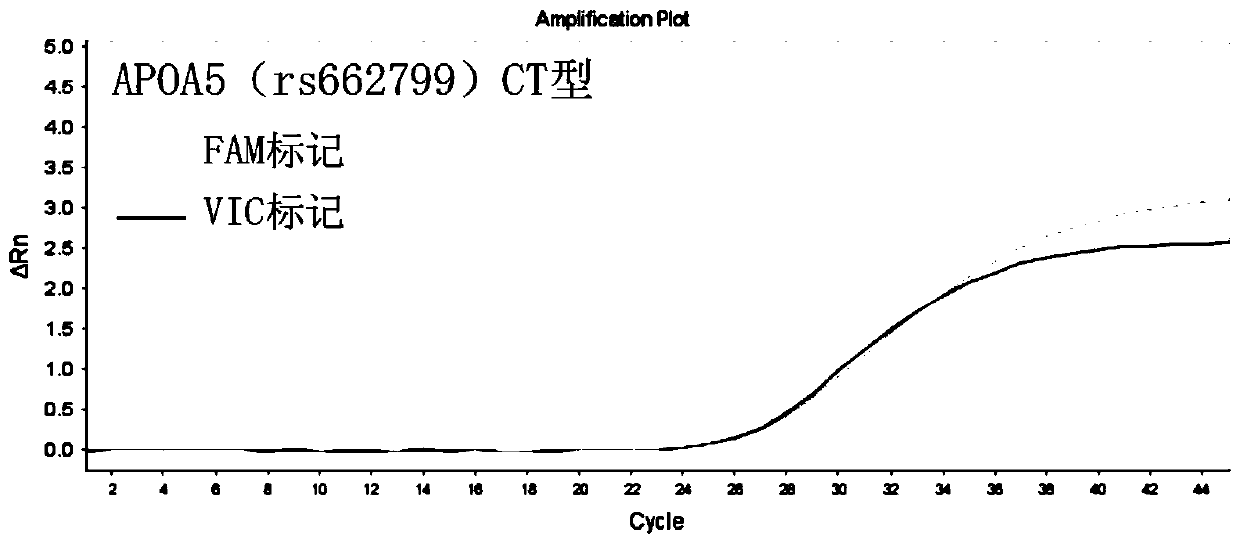
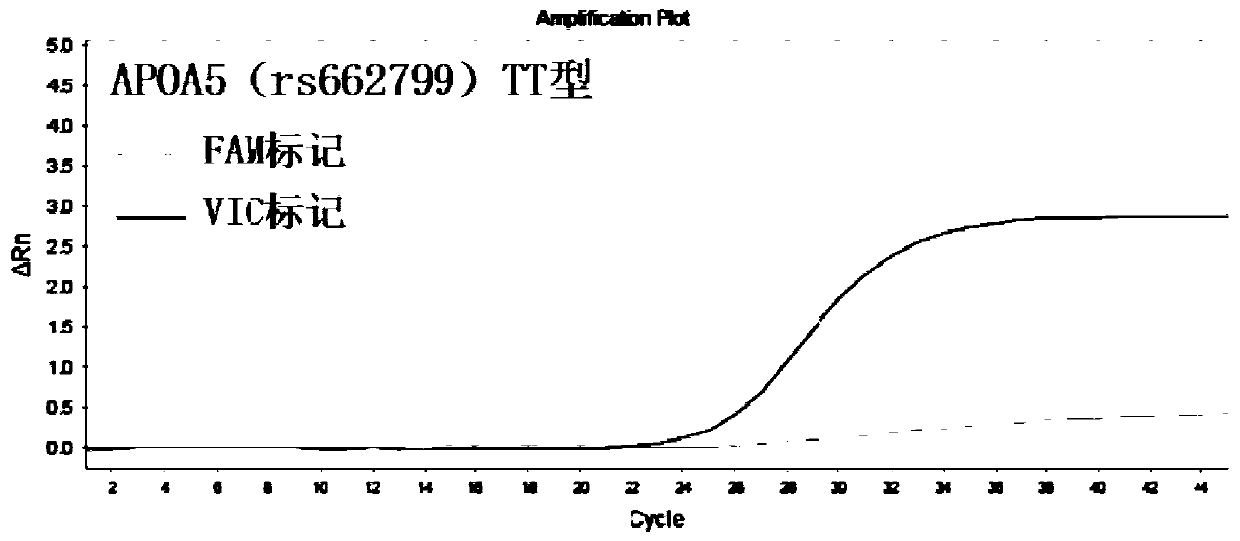
InactiveCN110295225AEasy to measureFast and accurate determinationMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Lovastatin
The invention discloses a primer combination used for guiding lovastatin drug individualized medication related gene detection. The primer combination includes specific primers for APOA5(rs662799), CETP(rs708272) and SLCO1B1(rs2291073) gene loci and Taqman probes. The invention further discloses a kit comprising the primer combination and an analysis method thereof. The primer combination has theadvantages of being easy to operate, economical, rapid in detection, high in accuracy, good in specificity and simple in result interpretation, rapid and accurate determination for the lovastatin drugindividualized medication related gene polymorphism is achieved, so that adverse reactions of clinical lovastatin drug common medication are reduced, the medical cost is lowered, and the social resources are saved.
Owner:南京派森诺基因科技有限公司
Primer set, kit and method for detecting cardiovascular disease-related gene polymorphisms
ActiveCN108004312BStrong specificityHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Medicine
The invention discloses a primer set for detecting cardiovascular disease related gene polymorphism. The primer set comprises primers aiming at following SNP loci of SLCO1B1 521C, SLCO1B1 521T, SLCO1B1 463A, SLCO1B1 463C, SLCO1B1 388A, SLCO1B1 388G, ABCA1 656A, ABCA1 656G, CYP2C9 1075C, CYP2C9 1075A, ABCB1 1236T, ABCB1 1236C, ABCB1 2677A, ABCB1 2677G, ABCB1 2677T, ABCG2 421C, ABCG2 421A, CYP3A4 1334C, CYP3A4 1334T, CYP3A4 653G, CYP3A4 653C, SLC10A1 800 and SLC10A1 800C. The invention further discloses a kit comprising the primer set and a gene polymorphism detecting method utilizing the primerset. The primer set has the advantages of being high in specificity, high in detecting sensitivity, and high in accuracy. The detecting method is high in specificity, high in sensitivity and low in cost, and simultaneous detection of multiple loci can be achieved.
Owner:德诺杰亿(北京)生物科技有限公司
Primer pair, probe and kit for detecting SLCO1B1 521T)C gene polymorphism
InactiveCN108977523AReduce design difficultyReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Fluorescence
The invention discloses a primer pair, a probe and a kit for detecting SLCO1B1 521T)C gene polymorphism. The kit comprises a primer and a fluorescence probe; the primer is designed for SLCO1B1 521T locus and has a base sequence shown as SEQ ID No.1 and SEQ ID No.2, wherein a 5'-base of the base sequence is equipped with an amino-modified group; the fluorescence probe is designed for SLCO1B1 521T locus and has the base sequence shown as SEQ ID No.3 and SEQ ID No.4, wherein one basic group in the base sequence is equipped with the amino-modified group. Compared with the prior art, the kit disclosed by the invention adopts the amino modified primer and probe, further limits the detection time and is capable of obviously shortening the time required by detection, reducing non-specific amplification, detecting SNPs and point mutation, reducing design difficulty and detection cost without influence on diagnosis accuracy, realizing a method for detecting mutation without opening a single reaction tube, extremely avoiding aerosol pollution, bringing convenience to clinic application and reducing the error caused by operation.
Owner:东莞博盛生物科技有限公司
Primer combination, kit and method for guiding detection of pravastatin individualized drug use related genes
InactiveCN110205370AReduce adverse reactionsEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Gene
The invention discloses a combination of primers for guiding detection of a pravastatin individualized drug use related genes, and is characterized in that the combination includes specific primers and probes for SLCO1B1 (rs4149015), HMGCR (rs17244841) and LDLR (rs1433099) gene loci. The invention also discloses a kit and a method. The kit has the advantages of simple operation, economy, fast detection, high accuracy, good specificity and simple result interpretation, and realizes rapid and accurate determination of polymorphisms of the pravastatin individualized drug use related genes, so asto reduce adverse reactions of common drug use of pravastatin in clinic, reduce medical cost and save social resources.
Owner:南京派森诺基因科技有限公司
Primer set and kit for detecting polymorphism of hyperglycemia drug metabolism related genes and application of primer set and kit
PendingCN112210598AReduce adverse reactionsAccurate typingMicrobiological testing/measurementDNA/RNA fragmentationRelated geneMedication use
The invention relates to the technical field of medicine and biology, and discloses a primer set and a kit for detecting polymorphism of hyperglycemia drug metabolism related genes and application ofthe primer set and the kit. The primer set comprises primers capable of amplifying at least one hyperglycemia drug metabolism related gene: CYP2C9, KCNJ11, PPARgamma, SLCO1B1, SLC22A1, SLC22A2, and APOE. The primer set can also comprise a sequencing primer for carrying out Sanger sequencing on at least one of the above genes. The primer set and kit can formulate different schemes for hyperglycemiapatients according to individual differences of genes, select appropriate hypoglycemic drugs, realize accurate typing and precise medication, thereby increase hypoglycemic effect and reduce adverse drug reactions. The preferred primer set has high sensitivity and specificity, and when the preferred primer set is used for genotyping genes related to hyperglycemia drug metabolism, the primer set has advantages of being accurate in qualitative analysis, high in specificity and the like.
Owner:PRECEDO PHARMA CO LTD
Compositions and methods for treating and preventing coronary heart disease
ActiveUS20130102582A1Accurate doseBiocideMicrobiological testing/measurementCoronary artery diseaseSLCO1B1
The invention pertains to a method of determining a statin dosage for an individual in need of treatment with a statin, comprising determining a SLCO1B1 genotype from a nucleic acid sample of the individual, said genotype comprising the presence or absence of the SLCO1B1-056 polymorphism, and determining an ApoE genotype or phenotype identifying an ApoE polymorphism selected from the group consisting of ApoE2, ApoE3, ApoE4, and any combination thereof, wherein the combination of a SLCO1B1 genotype identifying the presence of the SLCO1B1-056 C polymorphism and the ApoE genotype or phenotype identifying one of the ApoE3 / 4 or ApoE4 / 4 genotypes indicates the statin dosage.
Owner:BOSTON HEART DIAGNOSTICS
Primer and probe composition for detecting SLCO1B1 and APOE (apolipoprotein E) gene polymorphism and kit and method
InactiveCN111154860AMeet Mutation ScreeningMeet the needs of various mutation detection such as genotypingMicrobiological testing/measurementDNA/RNA fragmentationApolipoprotein e4SLCO1B1
The invention relates to a primer and probe composition for detecting SLCO1B1 and APOE (apolipoprotein E) gene polymorphism and a kit and a method. The primer and probe composition for detecting SLCO1B1 and APOE gene polymorphism comprises a first primer and a first probe and a second primer and a second probe, wherein the first primer and the first probe are used for detecting an SLCO1B1 locus; the second primer and the second probe are used for detecting an APOE locus; nucleotide sequences of the first primer are shown in SEQ ID No.1-4; nucleotide sequences of the first probe are shown in SEQ ID No.9-10; nucleotide sequences of the second primer are shown in SEQ ID No.5-8; and nucleotide sequences of the second probe are shown in SEQ ID No.11-12. The primer and probe composition has theadvantages of being high in detection flux and simple and convenient to operate.
Owner:深圳会众生物技术有限公司
Extraction-free reagent kit for detecting gene polymorphism of human APOE and SLCO1B1
PendingCN110951859AMeet detectionHigh amplification sensitivityMicrobiological testing/measurementSLCO1B1Nucleotide
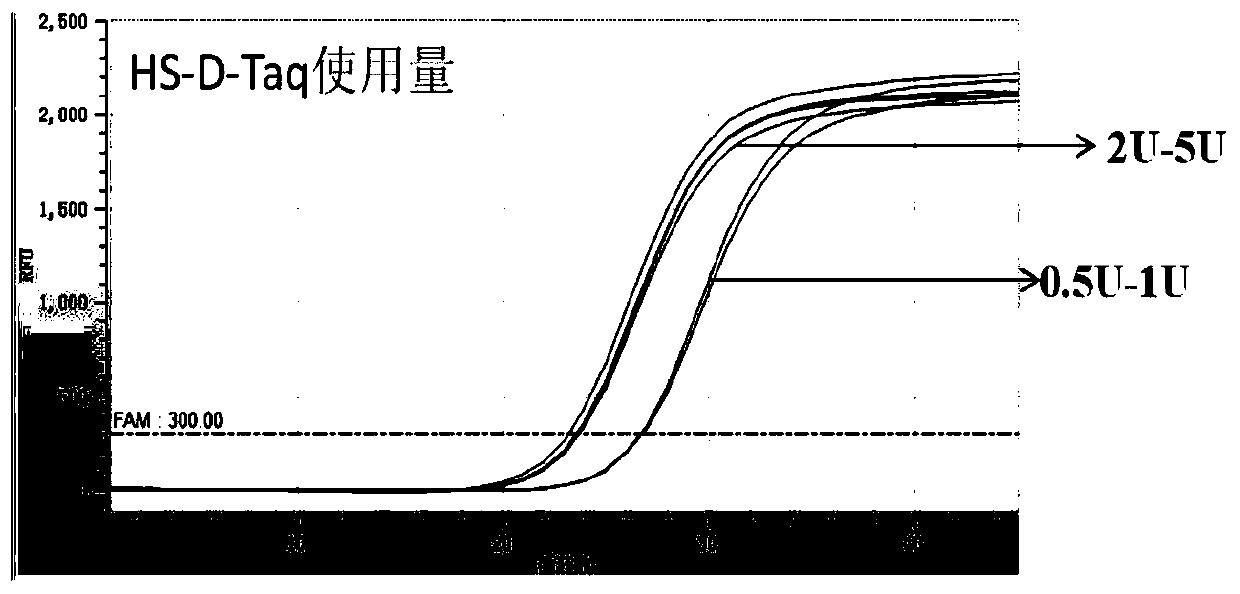
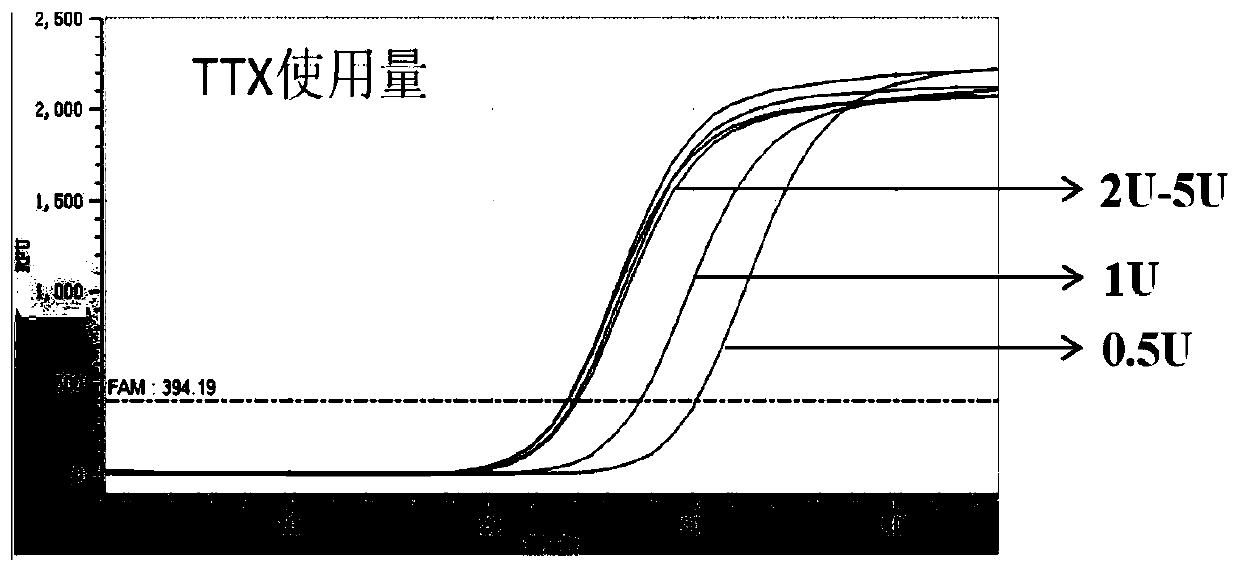
The invention discloses an extraction-free reagent kit for detecting gene polymorphism of human APOE and SLCO1B1. The reagent kit comprises main reaction liquor, ApoE primer and probe mixed liquor 1,ApoE primer and probe mixed liquor 2, SLCO1B1 primer and probe mixed liquor 1, SLCO1B1 primer and probe mixed liquor 2, positive quality control and feminine quality control, wherein the main reactionliquor comprises DNA polymerase, four kinds of deoxyribonucleotide, Mg2+, Tris buffer liquor and ultrapure BSA, and the DNA polymerase includes TTX enzymes and / or HS-D-Taq enzymes. The reagent kit can directly complete test on whole blood samples and swab samples through a unique amplification system, and a primer and probe sequence and system taking account of specificity and sensitivity, the detection is completed in four reaction system closed pipes, so that pollution can be avoided; and in addition, the feminine quality control and the positive quality control in the reagent kit can effectively monitor false positivity and validity of reagents and operations, and can effectively control pollution and monitor the whole experiment process.
Owner:苏州天隆生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com