Extraction-free reagent kit for detecting gene polymorphism of human APOE and SLCO1B1
A gene polymorphism and kit technology, applied in the field of molecular biology, can solve the problems of increasing clinical testing steps, man-hours, costs, operational errors, safety, etc., to control pollution and monitor the entire experimental process, prevent pollution, The effect of simple detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1. kit preparation
[0063] The main components of the main reaction solution are DNA polymerase, four deoxyribonucleotides, Tris buffer, Mg 2+ , Ultra-pure BSA.
[0064] 1. Main reaction formula:
[0065] 1.1: The raw material of DNA polymerase is Taq enzyme, the concentration is 2U-5U.
[0066] The raw material of DNA polymerase is purchased Taq enzyme, and the purchased suppliers include TOYOBO (product name TTX) and Xiamen Tongrenxin (product name HS-D-Taq). These two enzymes are highly resistant to interference and high amplification efficiency, and can be used for fluorescent PCR amplification.
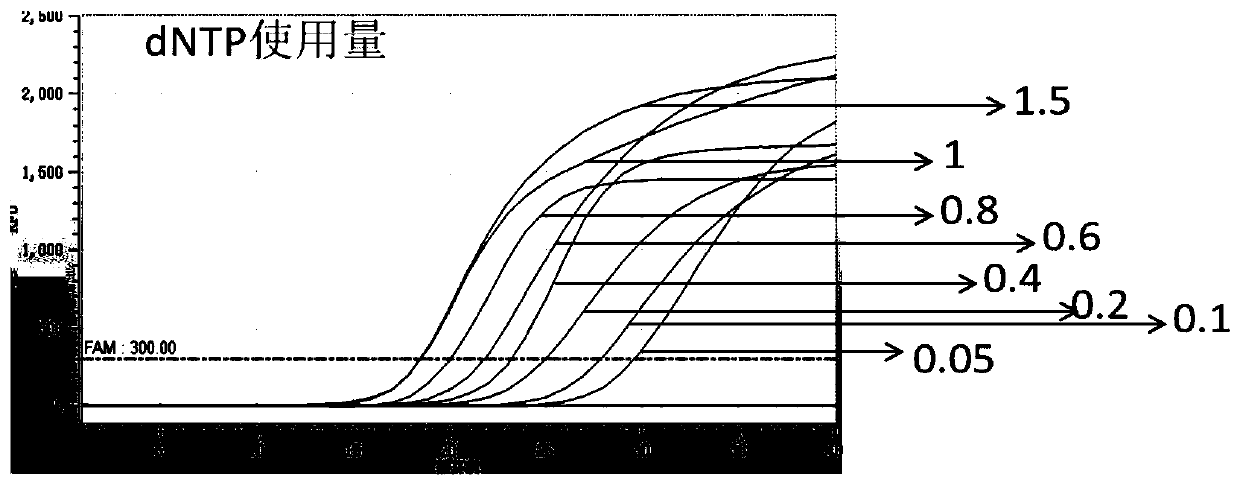
[0067] Enzyme usage test optimization in the platform system.
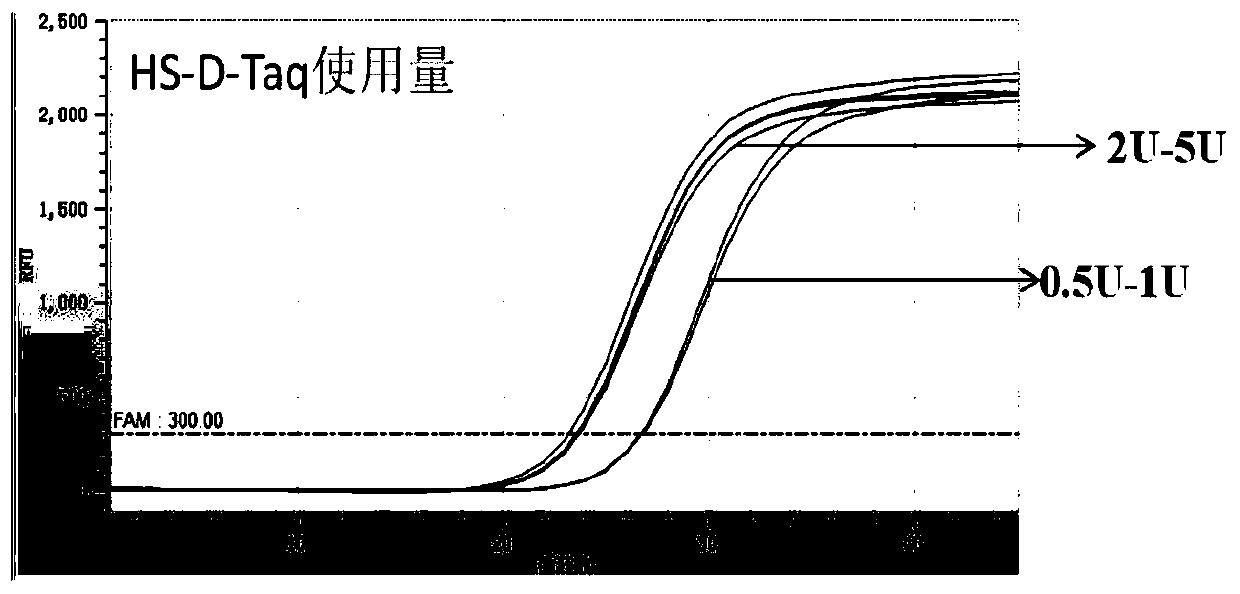
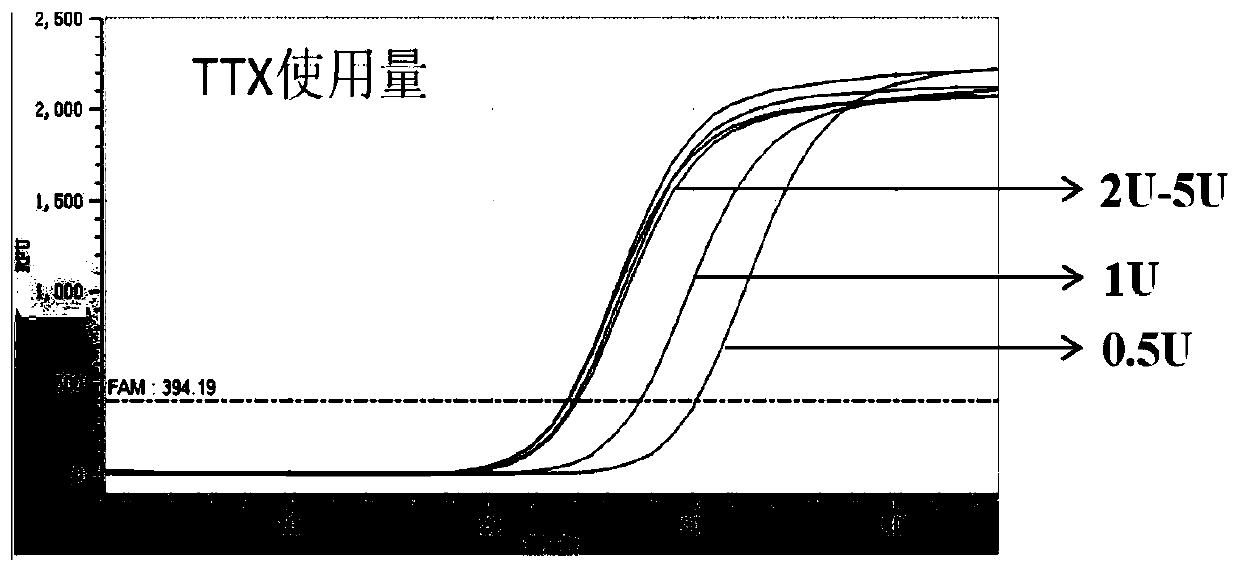
[0068] Test method: the total system is 20μl, the enzyme usage is 0.5U-5U, and the optimal enzyme usage is 2U-5U. For usage see figure 1 and figure 2 .
[0069] figure 1 It is the test result of optimizing the amount of HS-D-Taq enzyme. The amount of enzyme used is 0.5U-5U, which can be a...
Embodiment 2
[0160] Example 2. Kit whole blood sample test
[0161] Selected 9 cases of known APOE gene SNP sites rs429358 (c.388T>C) and rs7412 (c.526C>T), SLCO1B1 gene SNP sites (c.388A>G) and (c.521T>C) types Different whole blood samples were detected according to the operation steps in the detection method of the present invention, and the detection results can be found in Figure 7-Figure 24
[0162] The known genotypes of the samples and the genotypes detected by the kit of the present invention are shown in Table 15 below.
[0163] Table 15 Test result of kit of the present invention and known result contrast
[0164]
[0165] Conclusion: By analyzing 9 cases of known APOE gene SNP sites rs429358 (c.388T>C) and rs7412 (c.526C>T), SLCO1B1 gene SNP sites (c.388A>G) and (c.521T>C ) type of whole blood samples for extraction-free detection, and through the comparative analysis of the interpretation results in Table 15, the detection results of the kit of the present invention are...
Embodiment 3
[0166] Example 3. Comparison of detection results after nucleic acid extraction and extraction-free detection results
[0167] Experimental process: Select 4 known APOE gene SNP sites rs429358 (c.388T>C) and rs7412 (c.526C>T), SLCO1B1 gene SNP sites (c.388A>G) and (c.521T>C ) type whole blood samples were divided into 2 groups, one group of nucleic acid extraction and purification kit was used for nucleic acid extraction and purification, and the other group was directly detected according to the operation steps in the detection method of the present invention without nucleic acid extraction. For the detection results, see Figure 25-Figure 40 , see Table 16 for the specific interpretation results.
[0168] Table 16 Comparison of nucleic acid extraction and extraction-free detection results
[0169]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com