Maleimide propionyl piperazine heptamethine cyanine salt fluorescence carrier and preparation method and application thereof
A technology of maleimide propionylpiperazine and heptamethine salt, applied in the field of maleimide propionylpiperazine heptamethine salt fluorescent carrier and its preparation, can solve the problem of small Stokes shift, Light instability, fluorescent material leakage and other problems, to achieve high quantum efficiency and Stokes shift, ensure accuracy and precision, convenient and fast marking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
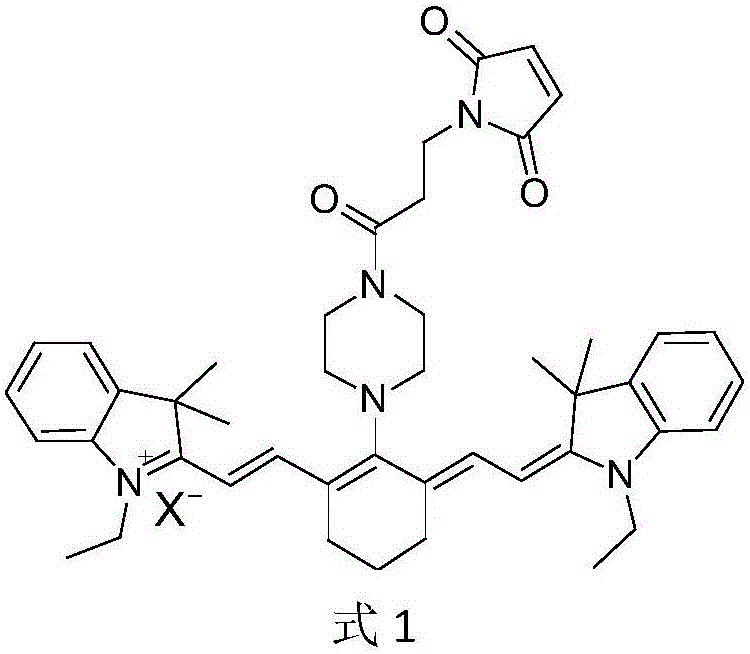
[0038] Example 1 The 2-(-2-(-2-(4-(3-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)propionyl)piperazine- 1-yl)-3-(-2-(1-ethyl-3,3-dimethylindol-2-ylidene)ethylene)cyclohex-1-en-1-yl)vinyl)- Synthesis of 1-ethyl-3,3-dimethyl-3H-indole iodide
[0039] method one:
[0040] This method synthetic example maleimide propionyl piperazine heptamethine iodide comprises the following steps:
[0041] S1: Add 327.01mg (3.80mM) of piperazine into a single-neck flask, add an appropriate amount of acetonitrile to dissolve, heat up to 40°C and stir, then weigh 240.40mg (0.38mM) of chloroheptamethine iodide, and add an appropriate amount of acetonitrile After dissolving, slowly add it dropwise into the piperazine solution, and monitor the reaction process by thin-layer chromatography. The reaction solution gradually changes from emerald green to blue. After 2-3 hours of complete reaction, remove the solvent by rotary evaporation, and use After extraction, the organic layers were combined, and the di...
Embodiment 2
[0047] Example 2 Covalent linkage of maleimide propionylpiperazine heptamethine iodide and mercaptododecaborane disodium salt (BSH)
[0048] Since the anion part of the maleimide propionyl piperazine heptamethine salt fluorescent carrier referred to in the present invention has no essential influence on the covalent chemical connection performance of the carrier, this example is mainly based on the maleimide propionyl piperazine Heptamethine iodide is the representative (code name CyP).
[0049] In this embodiment, the classic radioactive boron neutron capture therapeutic drug mercaptododecaborane disodium salt (BSH) containing a sulfhydryl group is selected as a nucleophile, and is covalently bound to the maleimide propionyl piperazine heptamethine salt Above, the formation of copolymer dodecaborane maleimide propionyl piperazine heptamethine sulfide salt (code name BS-CyP). This experiment proves that the maleimide propionylpiperazine heptamethine salt-based fluorescent car...
Embodiment 3
[0051] Embodiment 3 physicochemical properties experiment
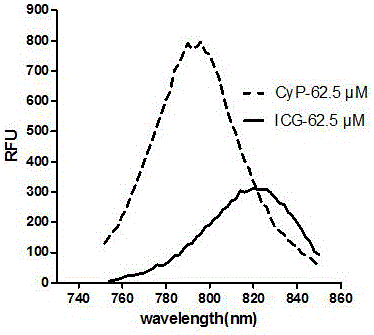
[0052] Because the anion portion of the near maleimide propionylpiperazine heptamethine salt of the present invention has no essential influence on the fluorescence performance of the carrier, this example is mainly based on the maleimide propionyl piperazine heptamethine iodide (CyP) as the representative.
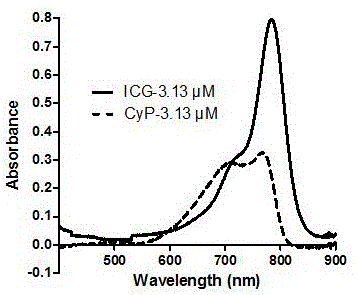
[0053] (1) Absorption spectra of near-infrared cyanine dyes ICG and CyP
[0054] ICG and the maleimide propionylpiperazine heptamethine iodide prepared in Example 1 were dissolved in methanol respectively to prepare a stock solution with a concentration of 1 mM, diluted to 3.13 μM, and scanned the ultraviolet absorption spectrum respectively to measure Its UV-Vis spectrum was obtained as figure 1 shown. Depend on figure 1 It can be seen that the maximum absorption wavelength of ICG in methanol is 790nm, with a small shoulder at 710nm. CyP presents obvious double absorption peaks, the maximum absorption wavel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com