Particle agglutination detection method and device
A particle and particle suspension technology, applied in the direction of measuring devices, biochemical equipment and methods, microbial measurement/testing, etc., can solve the problems of bulky test tubes, appearance misunderstanding, complexity, etc., to avoid false positive results, easy to use, Effects of Low Margin of Error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0137] In another embodiment of the invention, a device is provided having multiple test areas (eg, for blood typing of a single sample). The device can be constructed so that the test sample, and optionally the wash, is dispersed throughout all of the test areas in one step. Such an embodiment can be achieved through various design points, such as: (a) covering all test areas with a single piece, preferably hydrophilic mesh; (b) by placing non-absorbent A permanent covering creates a capillary space on the test area.
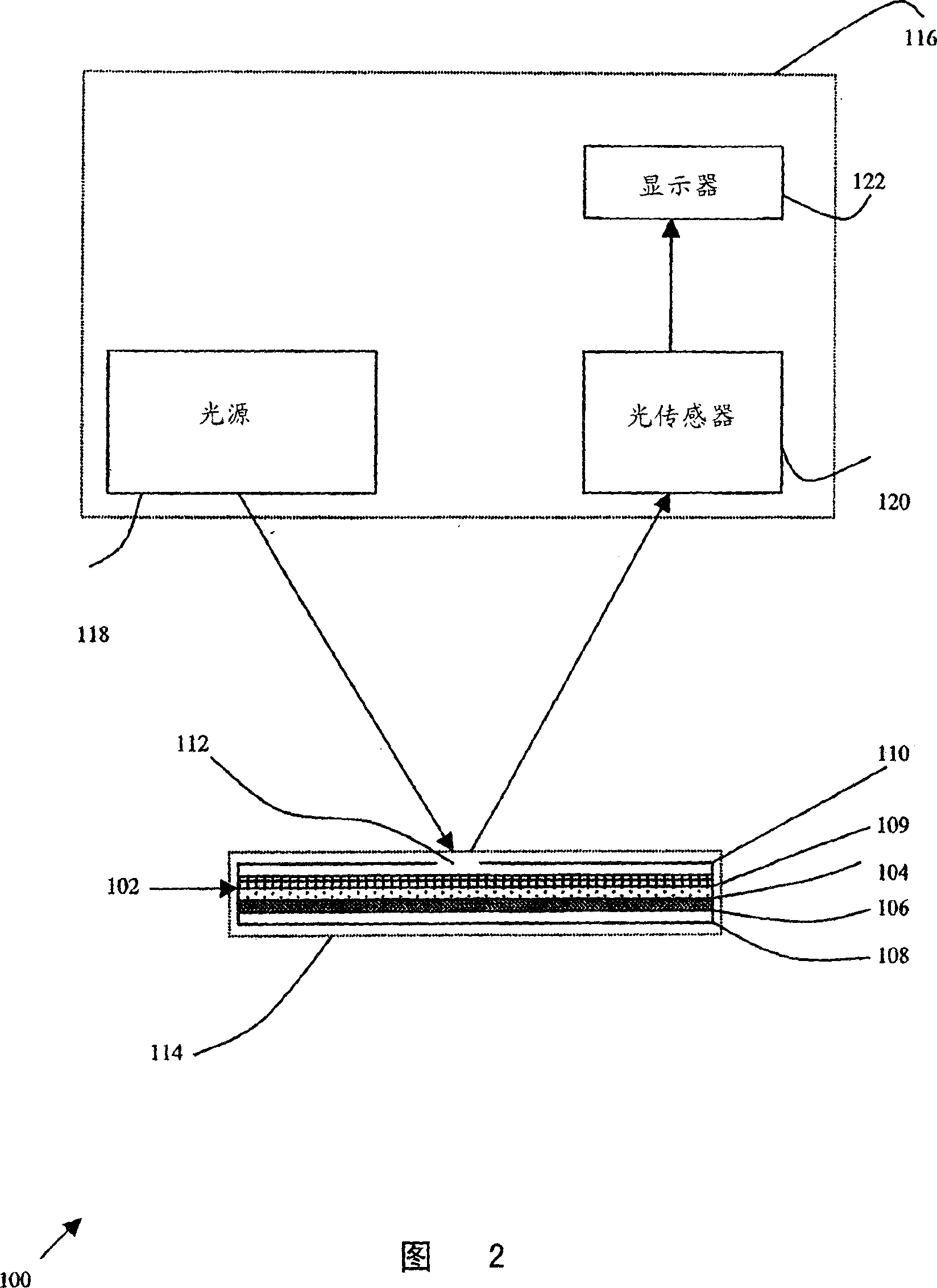
[0138] The device may further include an optional meter 116 that can interpret test results without human visual identification. Meter 116 preferably receives card 102 to enable meter 116 to measure a marker or reporter for detecting agglutination. Optionally, meter 116 is an optical meter and measures the amount of light reflected or scattered from or transmitted through the location where the sample is placed and determines the result accordingly. In its m...
Embodiment
[0142] The following examples are illustrative implementations of the methods and systems described herein. These examples are not intended to be limiting in any way.
[0143] Example 1 - Lotion
[0144] The washes used to form this example were:
[0145] Dulbecco's phosphate buffered saline (PBS) was obtained from Biological Industries, Beit Ha'emek, Israel.
[0146] Solutions were made of PBS diluted 1:1 in water with 4% w / v polyethylene glycol (PEG) 15000-20000 MW (Fluka) and 0.3% w / v dextran sulfate sodium salt (Amersham Biosciences).
[0147] A. Dulbecco's phosphate buffered saline (PBS) with 0.001-0.01% w / v polyoxyethylene-10-tridecyl ether (Sigma).
Embodiment 2
[0148] Embodiment 2-blood type identification
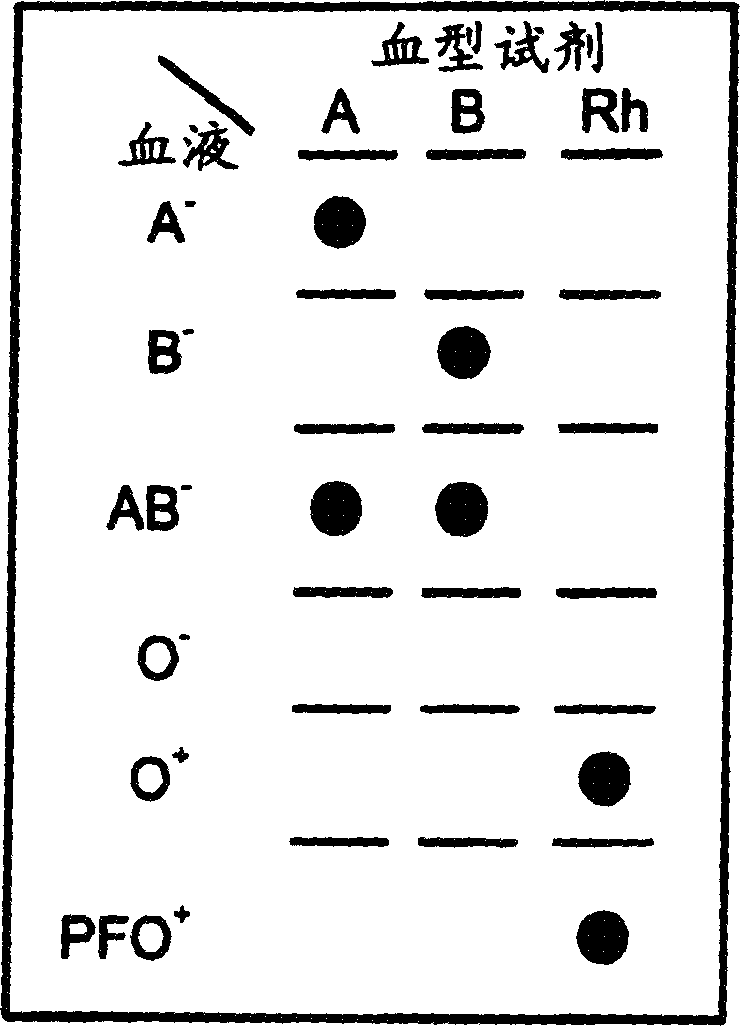
[0149] A 0.5 x 0.5 cm piece of Ahlstrom #142 filter paper was placed on an absorbent pad. 2 μL of whole blood was pipetted into the center of the filter, followed by 2 μL of anti-blood typing reagent (GammaBiologicals Inc., Hosuton, TX, USA). The filter was then washed with a few drops (from a dropper bottle) of washing solution and dried. Alternatively, use 4 mm diameter round Ahlstrom #142 filter paper, 10 μL blood and 10 μL antiblood group reagent, followed by a few drops of Wash Solution C from Example 1.
[0150] This test was repeated with multiple blood samples of various blood types (sourced and pre-tested by Central Blood Services, Israel Red Magen David, Tel Hashomer, Israel), each of which was tested with anti-A, anti-B, anti- AB and anti-D (=Rh) blood group reagents were tested. A-blood produces red dots only with anti-A and anti-AB reagents. B-blood was spotted with anti-B and anti-AB reagents. AB-blood reacts w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com