Patents
Literature
31 results about "Clinical laboratory test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical laboratory testing plays an essential part in the delivery of quality health care. A physician or other clinician orders lab tests to diagnose, treat, manage, or monitor a patient’s condition. The process begins with the collection of a sample of blood, tissue, or other biological matter from the patient,...
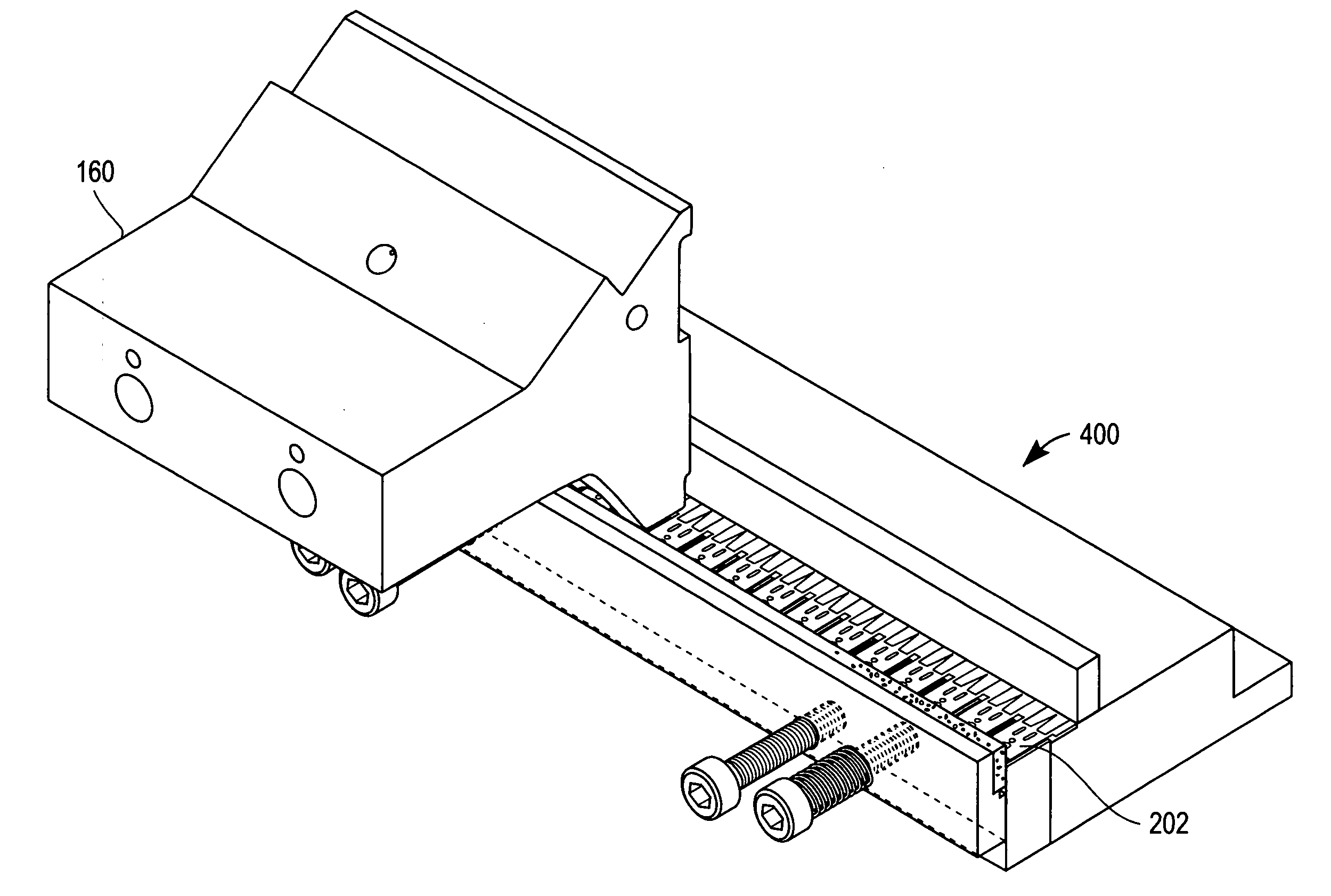
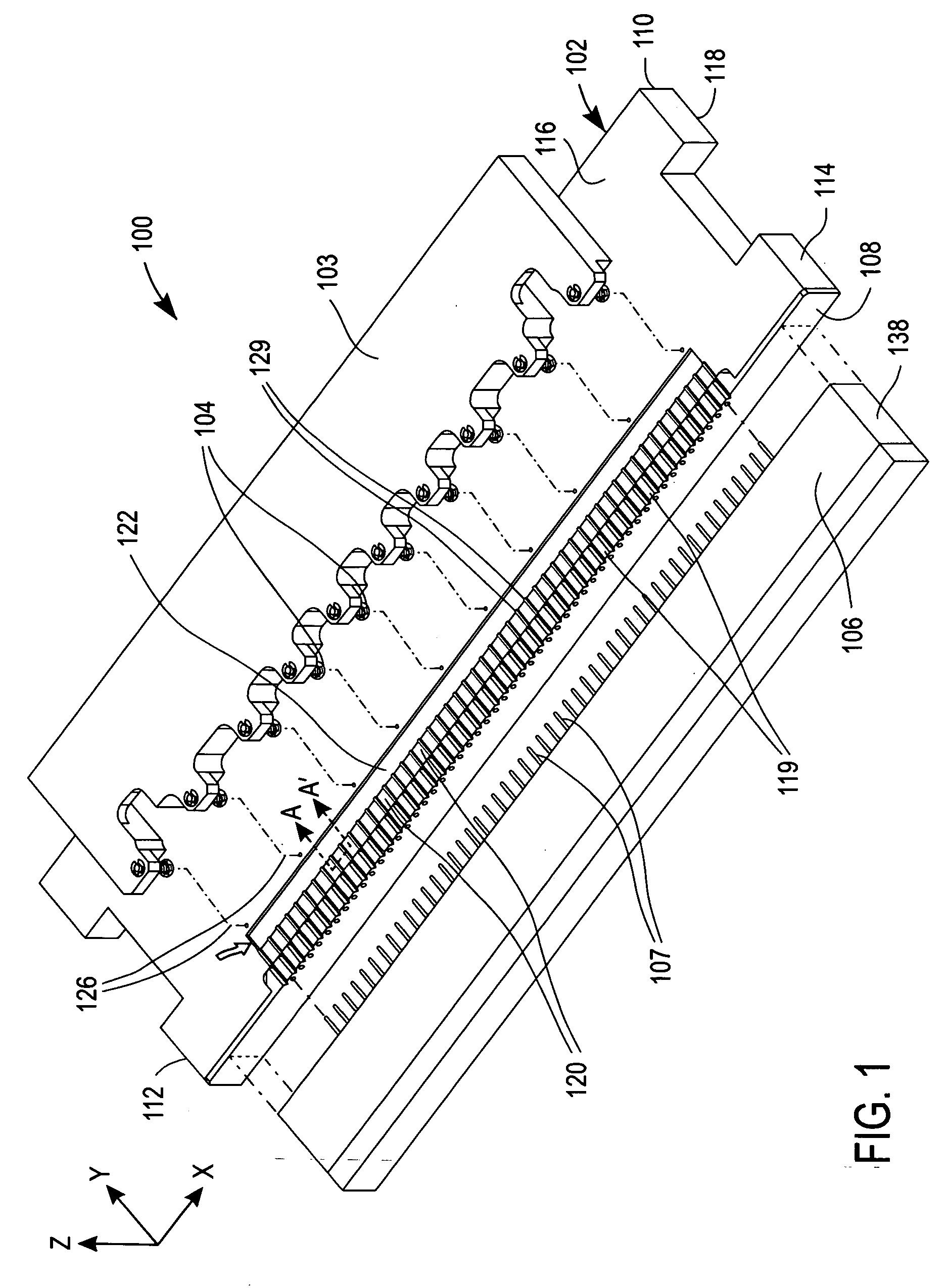
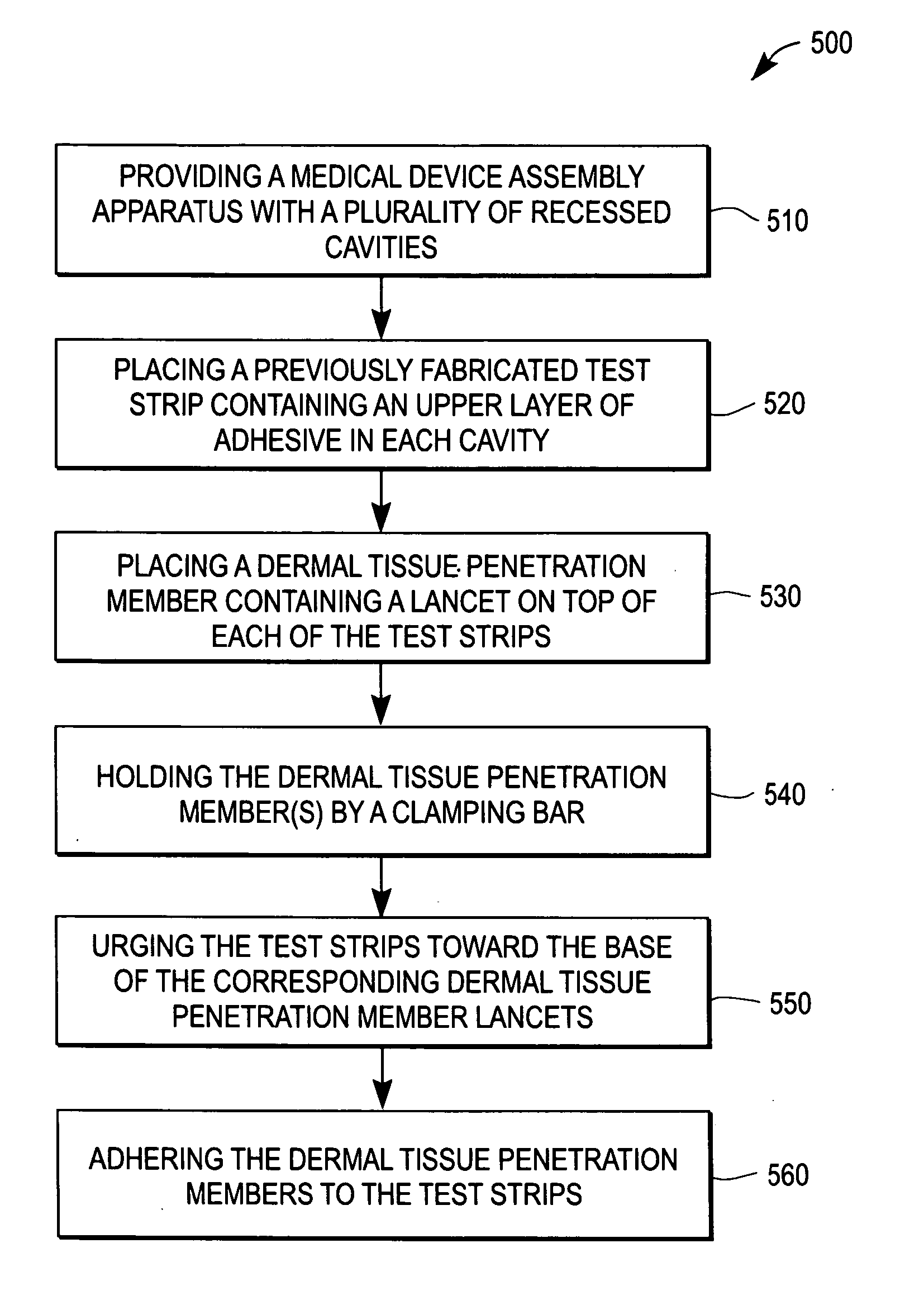
Apparatus for the manufacture of medical devices
InactiveUS20060006574A1Manual label dispensersAdhesive processes with adhesive heatingMedical equipmentAnalyte
The determination of analyte concentration in physiological samples is of ever increasing importance to today's society. Such assays find use in a variety of applications, including clinical laboratory testing, home testing, etc., where the results of such testing play a prominent role in the diagnosis and management of a variety of disease conditions. An apparatus is described herein which may be used for the manufacture of medical devices which include an integrated lancet and sensor.
Owner:LIFESCAN INC
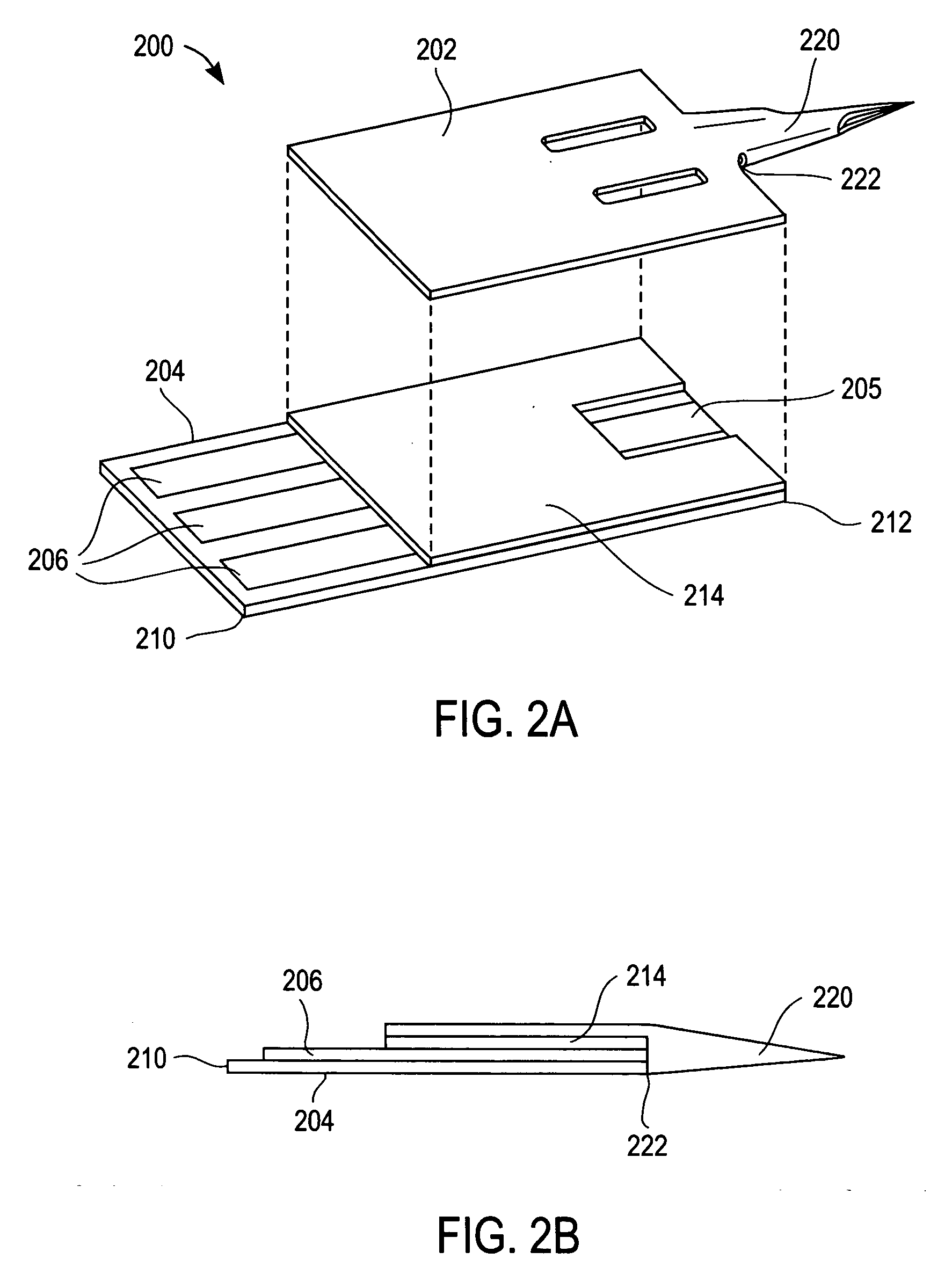
Method of manufacturing integrated biosensors
InactiveUS20060000549A1Minimize movementGuaranteed adhesionManual label dispensersAdhesive processes with adhesive heatingAnalyteAssay
The determination of analyte concentration in physiological samples is of ever increasing importance to today's society. Such assays find use in a variety of applications, including clinical laboratory testing, home testing, etc., where the results of such testing play a prominent role in the diagnosis and management of a variety of disease conditions. In the present application, a method is described which may be used for the manufacture of medical devices which include an integrated lancet and sensor.
Owner:LIFESCAN INC
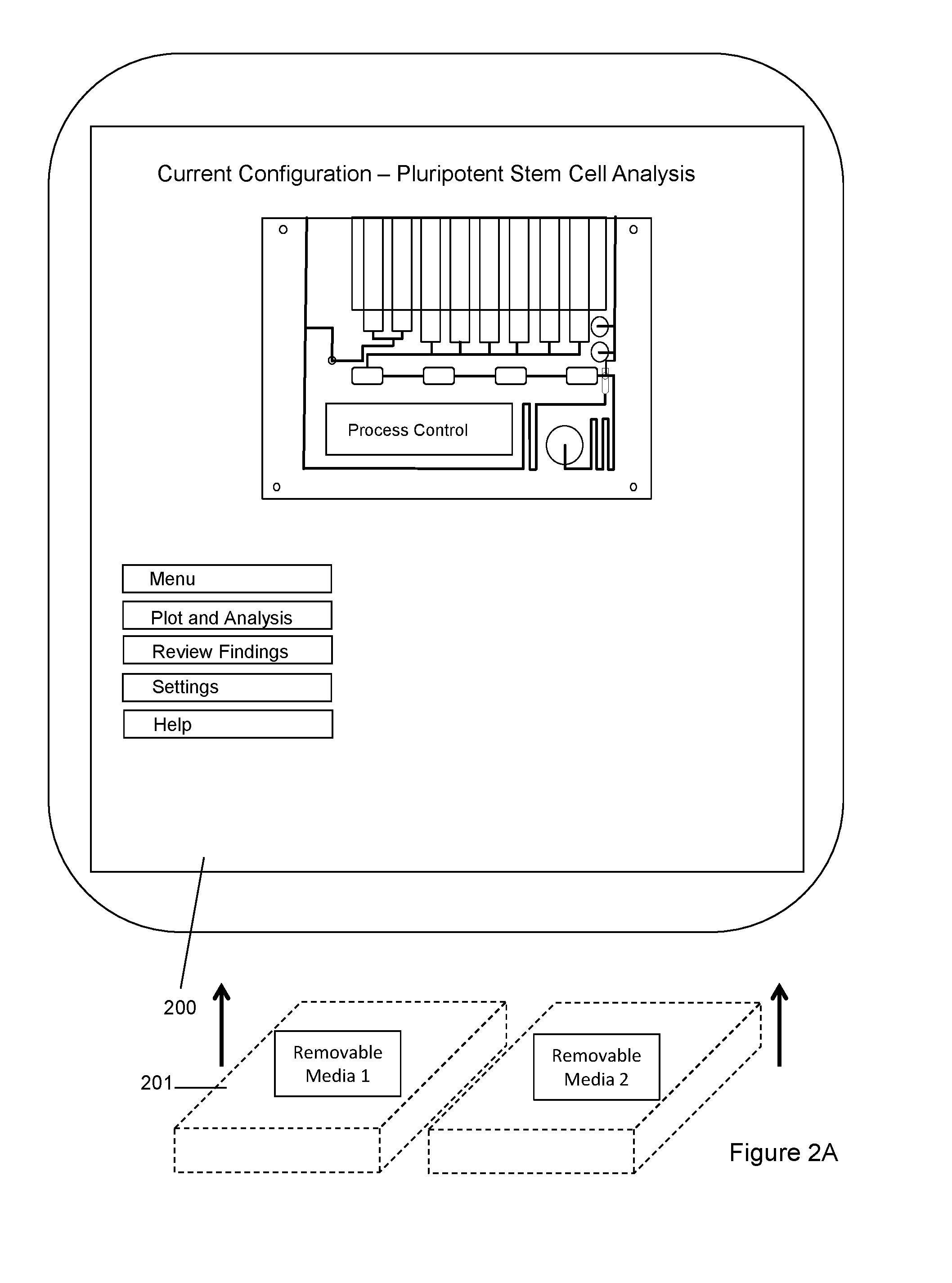
Dynamic Lab on a Chip Based Point-Of-Care Device For Analysis of Pluripotent Stem Cells, Tumor Cells, Drug Metabolites, Immunological Response, Glucose Monitoring, Hospital Based Infectious Diseases, and Drone Delivery Point-of-Care Systems
The invention provides for a novel dynamically configurable point-of-care device for clinical diagnostics and research for analysis of activity associated with pluripotent stem cells, tumor cells, drug metabolites, immunological response, glucose monitoring, cardiovascular diseases, liver cell therapy, cell-cell signaling, epidemic outbreaks, hospital based infectious diseases, pathogens, germ cells, pharmacological compounds, oxidation reduction, microscopy, tomography, flow cytometry, clinical lab testing, and for providing immunoassays, ELISA, electrophoresis, PCR, chromatography, and other laboratory functions. The device comprises a biochemical processing module further comprising a processor and at least one controller, receiving microfluidic elements, sensors, software scripts, an electrically operated interface, flow ports, a user interface, memory, and a communications link, configurable based on analysis of patient data. The invention further provides for multiple-criteria decision analysis for hospital administrators, a wearable device, mobile medical device, molecular electronics configuration, touchscreen recognition, data analytics application, and a drone delivery based point-of-care system.
Owner:PATEL NILESH
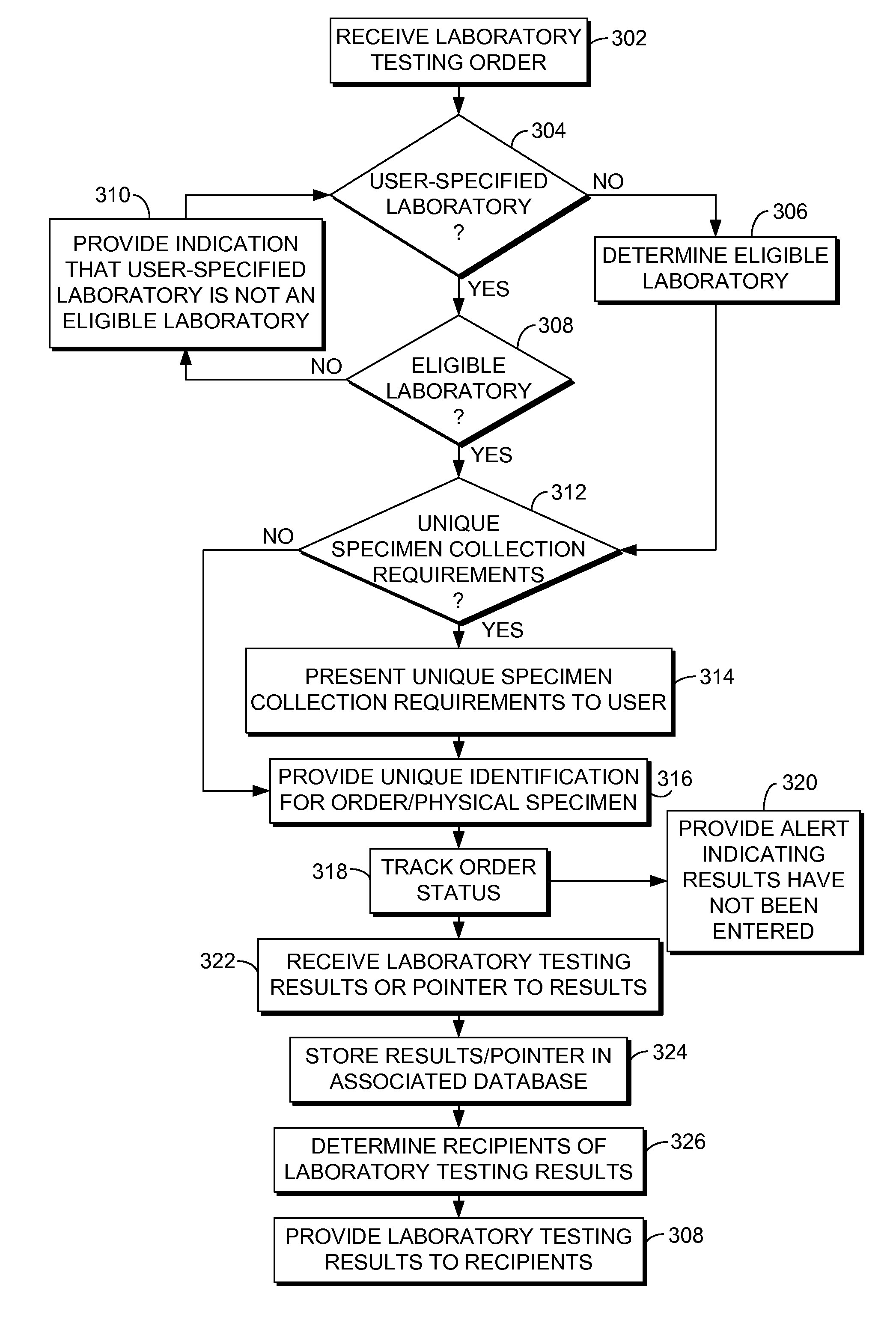
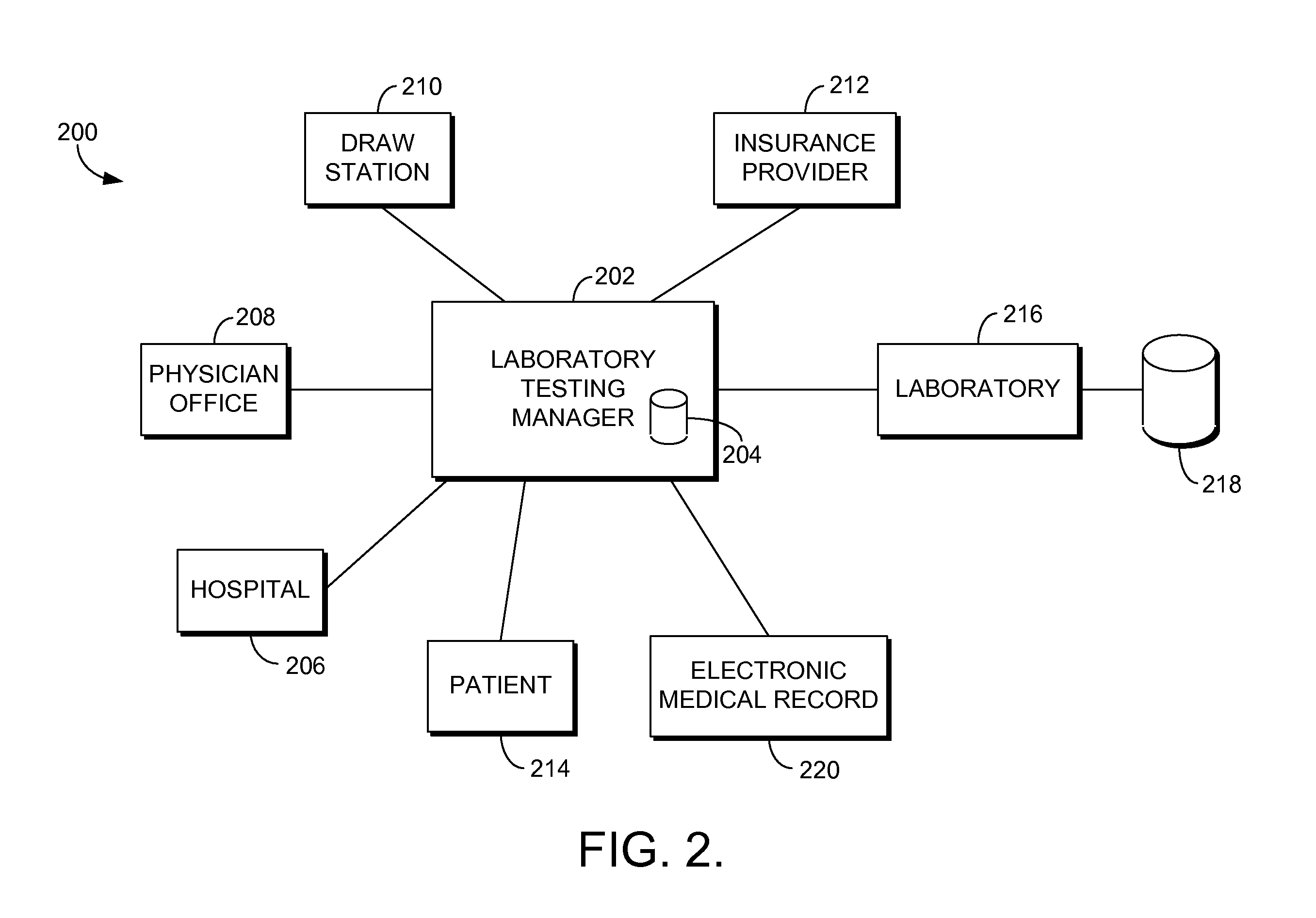
Automated laboratory test ordering and result tracking
InactiveUS20070294103A1Data processing applicationsComputer-assisted medical data acquisitionComputerized systemTest order
Computerized systems and methods are provided for coordinating clinical laboratory testing from initial order entry to results notification. A laboratory testing manager provides a centralized conduit for interfacing entities placing orders for laboratory testing and laboratories performing testing. Entities, such as healthcare providers, patients, and the like, may access the laboratory testing manager to enter orders for laboratory testing. The laboratory testing manager may determine an eligible laboratory to perform the laboratory testing based on information provided in each order, such as the type of testing requested and insurance coverage information. After a laboratory performs testing specified in an order, the laboratory may enter the testing results, which are routed to recipients indicated for the results.
Owner:CERNER INNOVATION
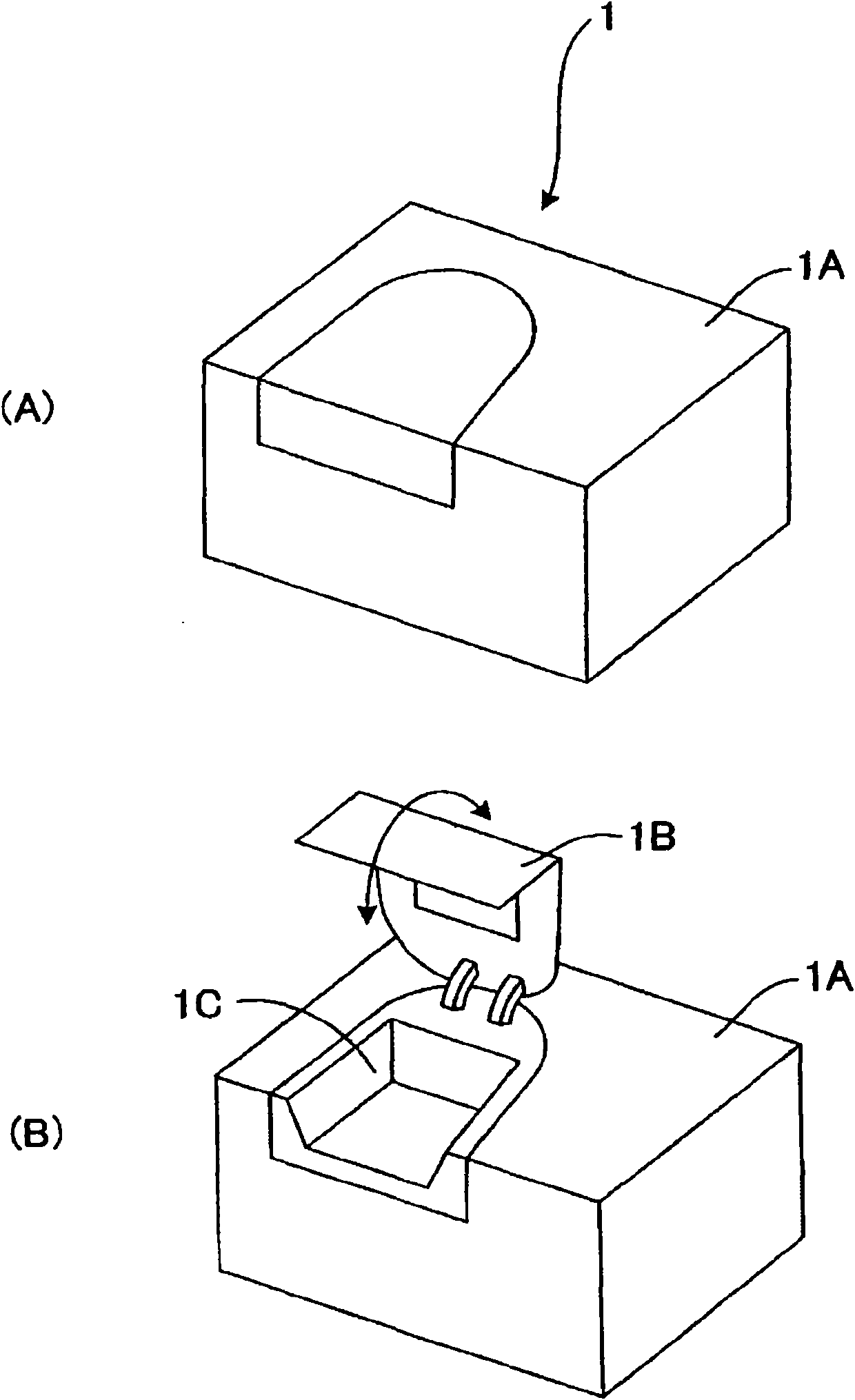
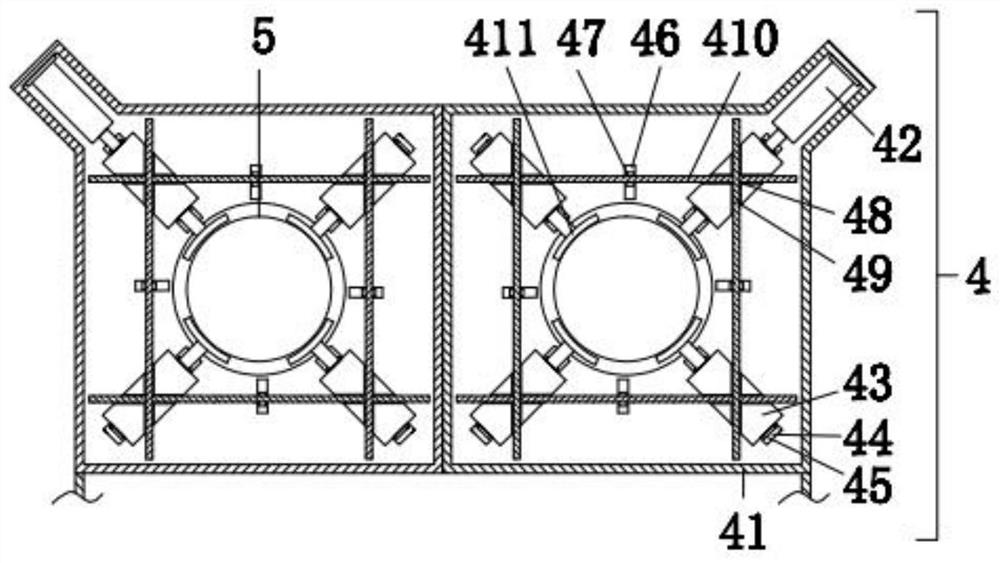
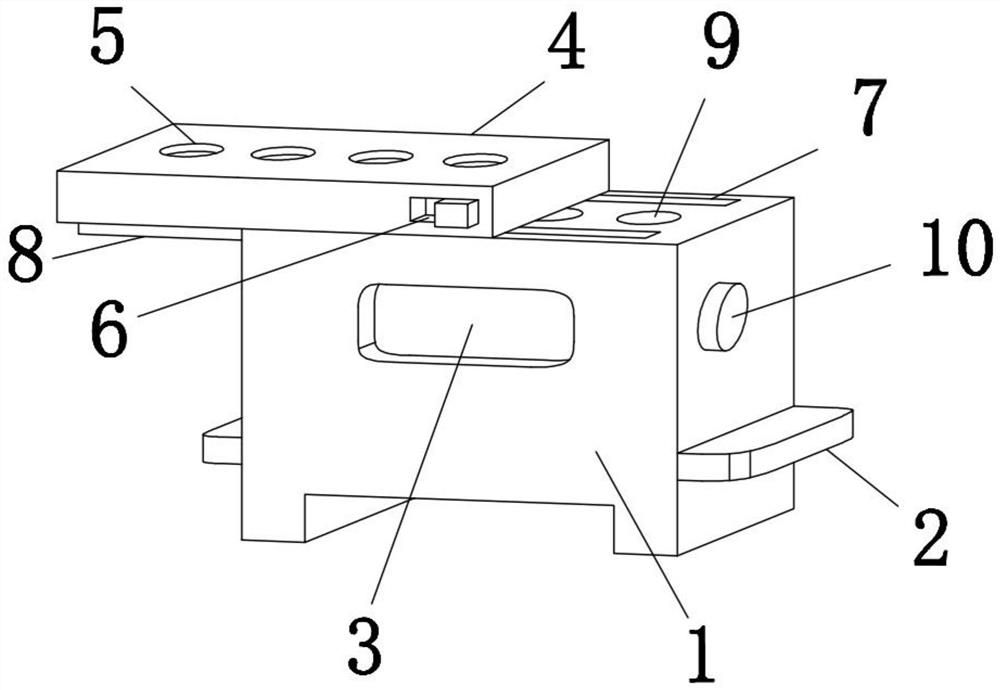
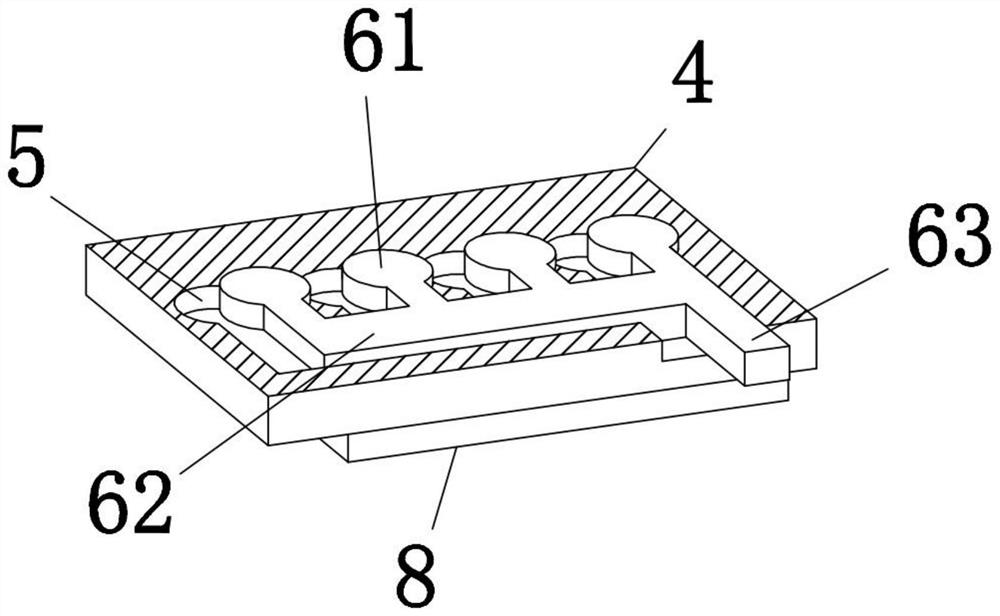
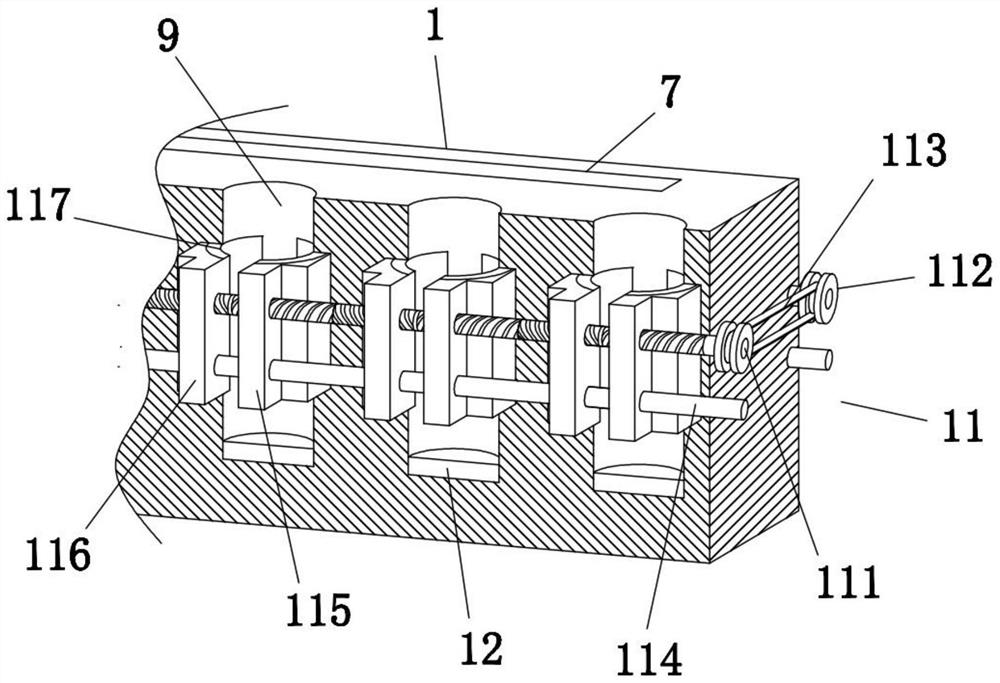
Clinical laboratory test apparatus
InactiveCN102004159AInexpensive and reliable to installCentrifugesMaterial analysisClinical examLocking mechanism
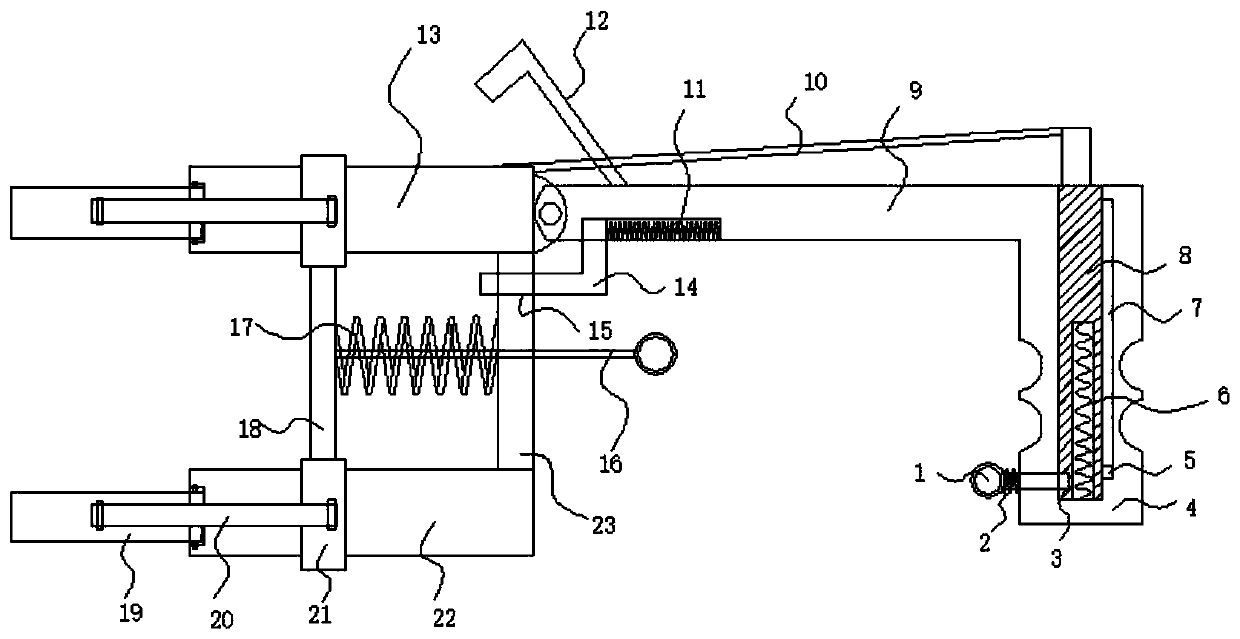
The present invention relates to a clinical laboratory test apparatus capable of simply and quickly attaching and detaching a microchip. The clinical laboratory testing apparatus comprises a microchip having a measuring cell that holds a sample liquid; a rotation body; a rotation drive mechanism that rotates the rotation body; a lock mechanism that locks the microchip on the rotation body; a measurement room that holds the microchip and the rotation body and that has an attachment and detachment opening; a protection cover that closes the attachment and detachment opening; a light source that irradiates the measuring cell; and a light receiving unit that receives the light, wherein a centrifugal separation processing of a specimen in the sample liquid is performed in the microchip by the rotation drive mechanism rotating the rotation body, and wherein the protection cover has a pressing-down member that presses down a wall face of the microchip when the protection cover is closing the attachment and detachment opening.
Owner:USHIO DENKI KK
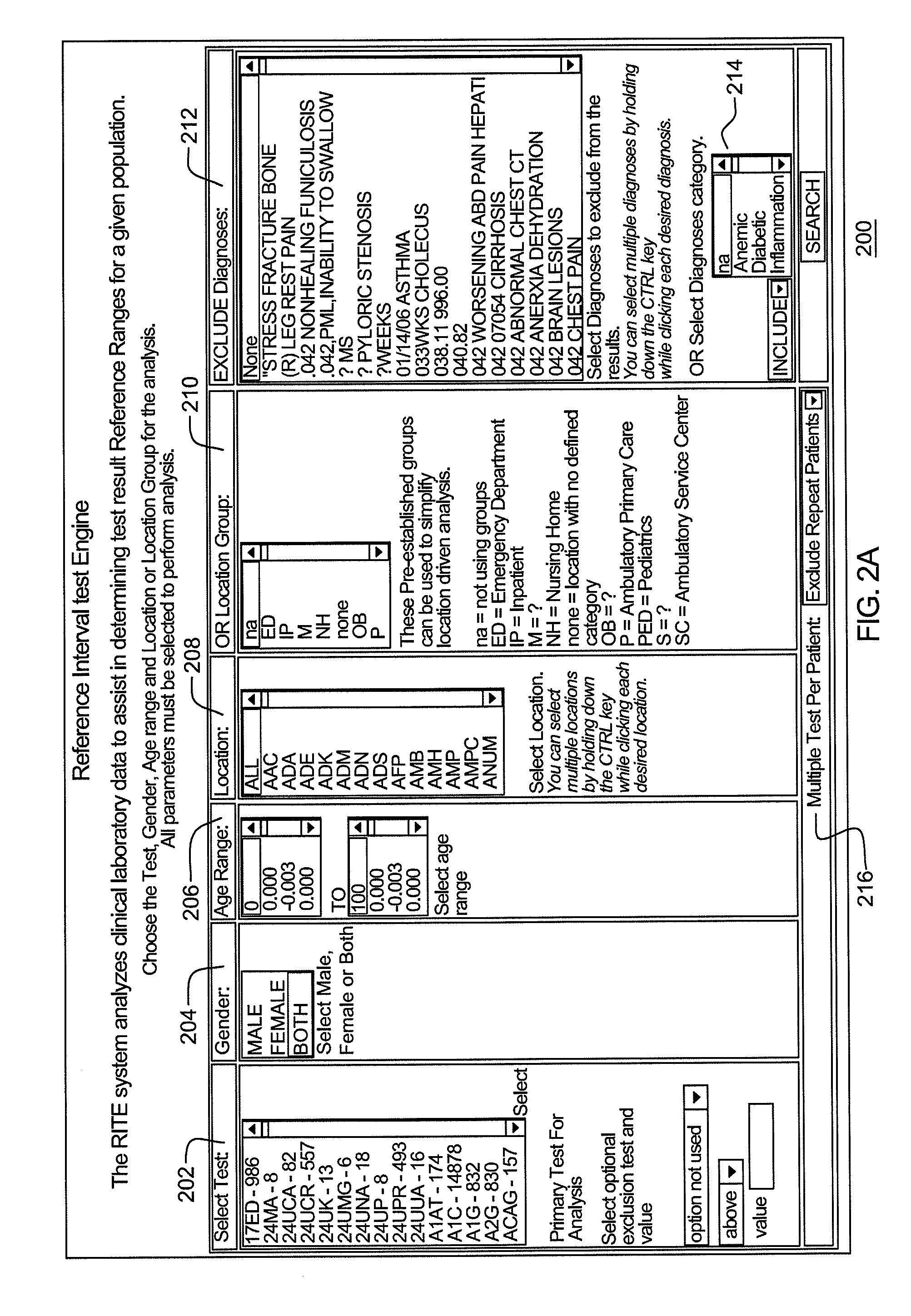
Performing data analysis on clinical data
ActiveUS20080294350A1Medical data miningComputer-assisted medical data acquisitionDatum referenceStatistical analysis
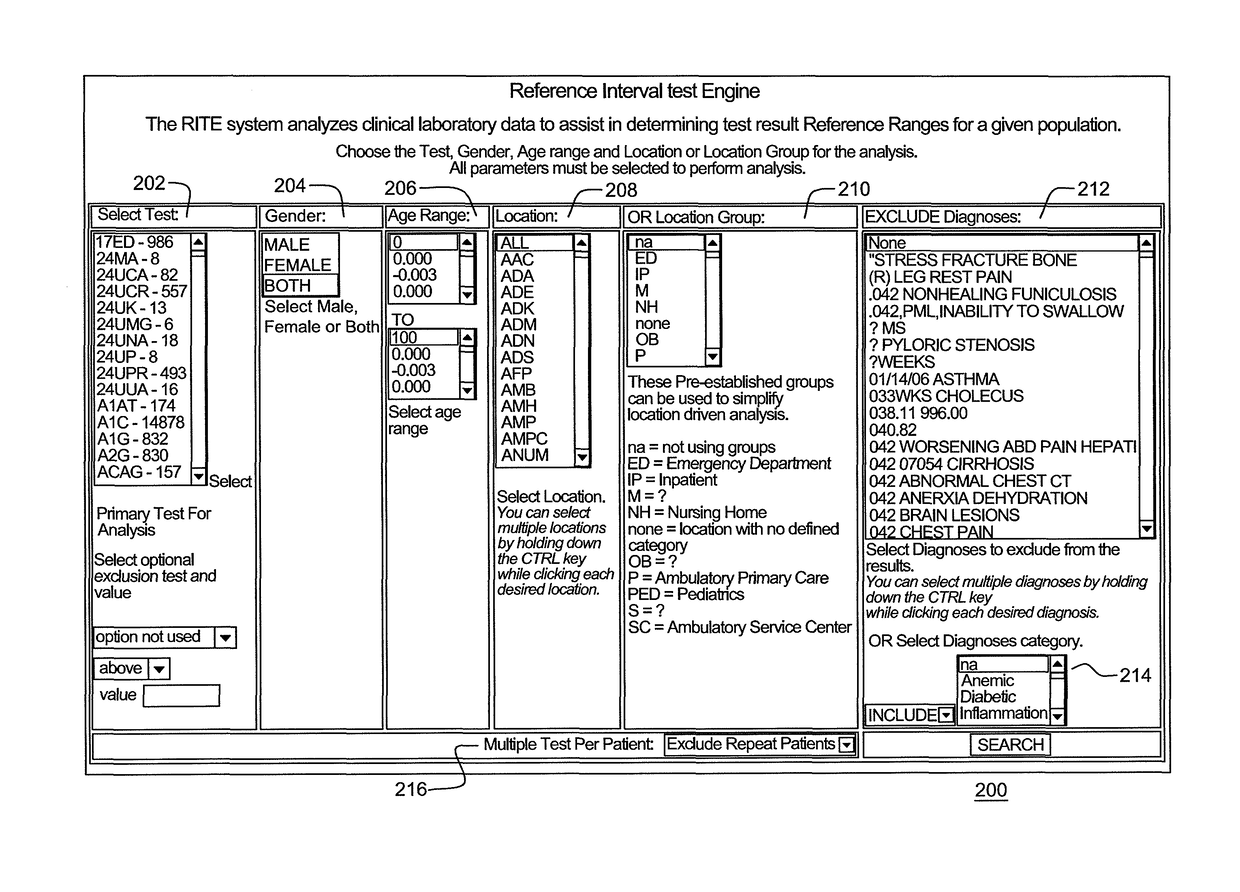
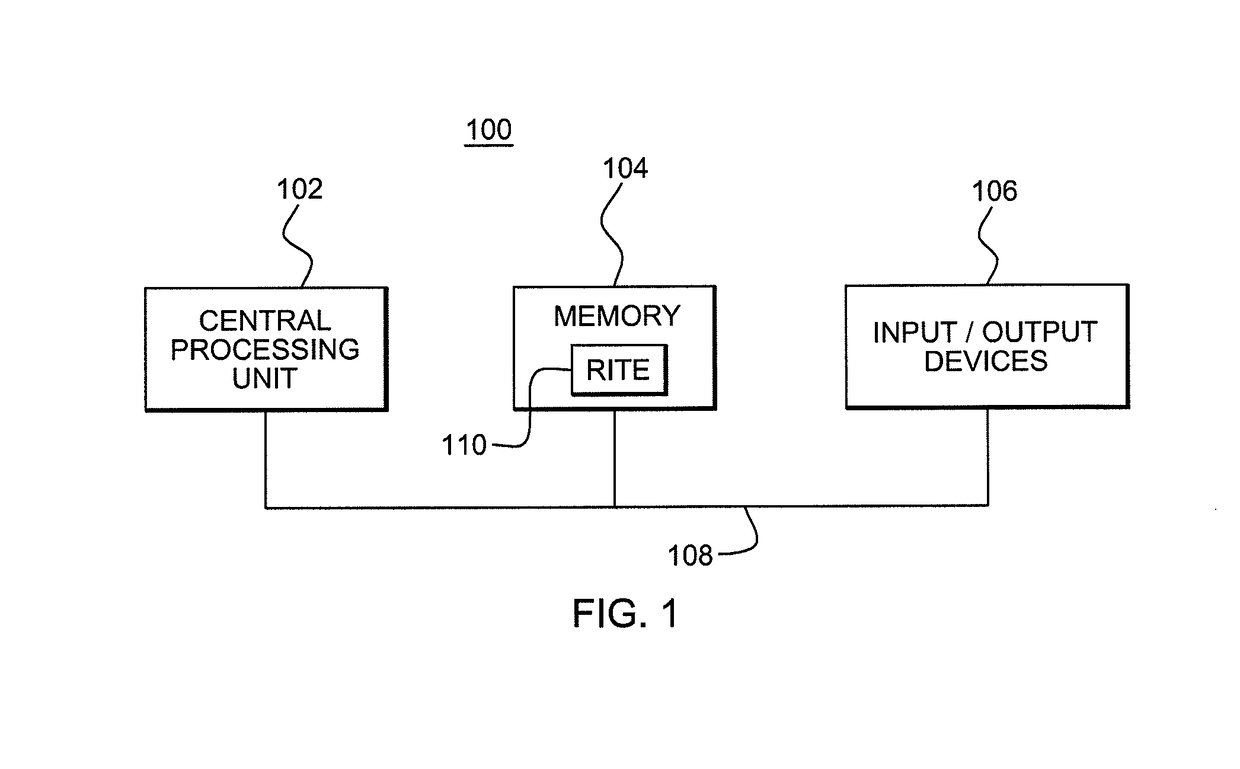
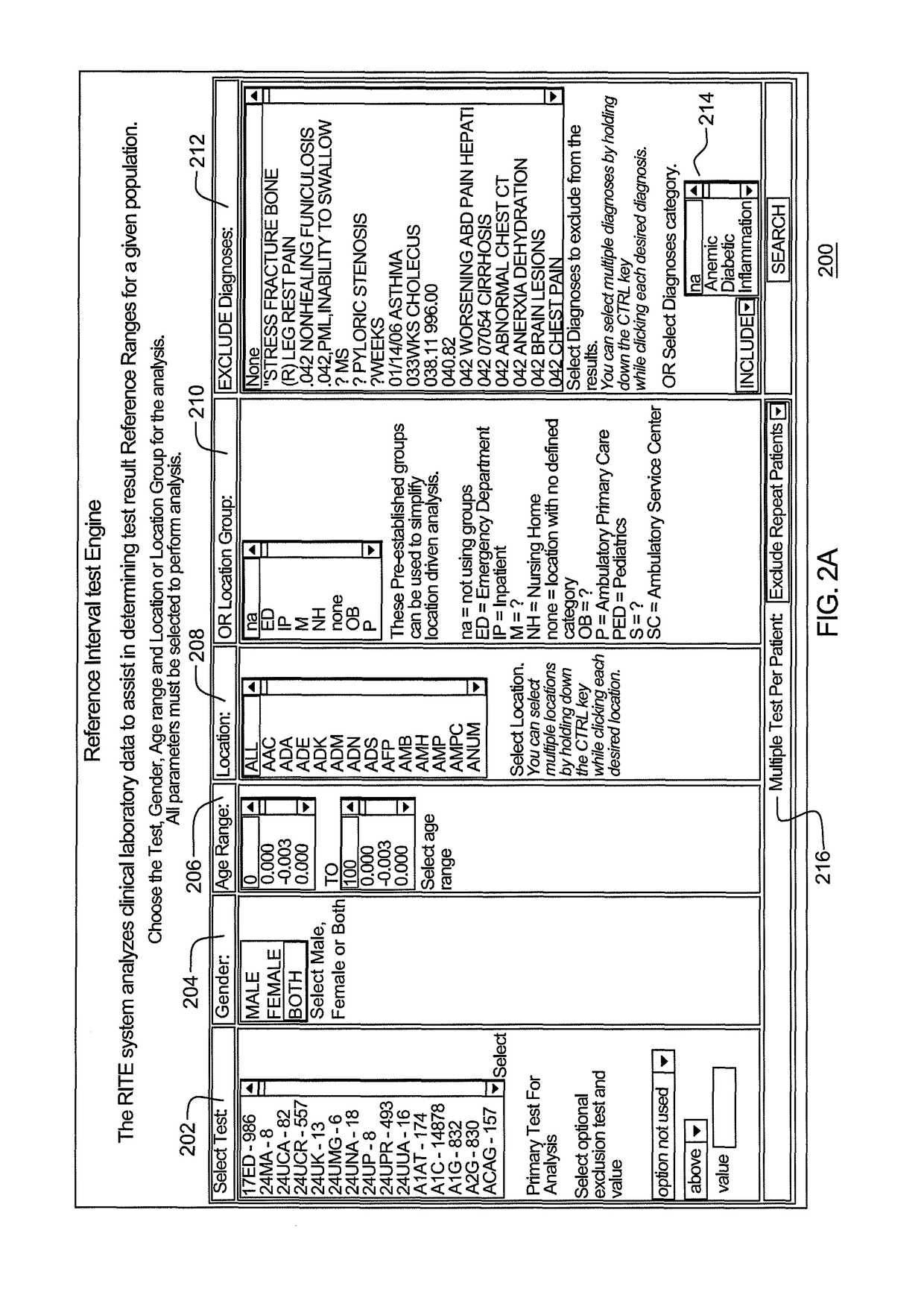
Reference intervals are established and / or validated based on existing clinical data and exclusion criteria, such as diagnosis coding. A Reference Interval Test Engine is designed to statistically analyze large volumes of existing clinical lab test results to establish and evaluate reference intervals for specific population subgroups and / or to provide other applications.
Owner:ALBANY MEDICAL COLLEGE
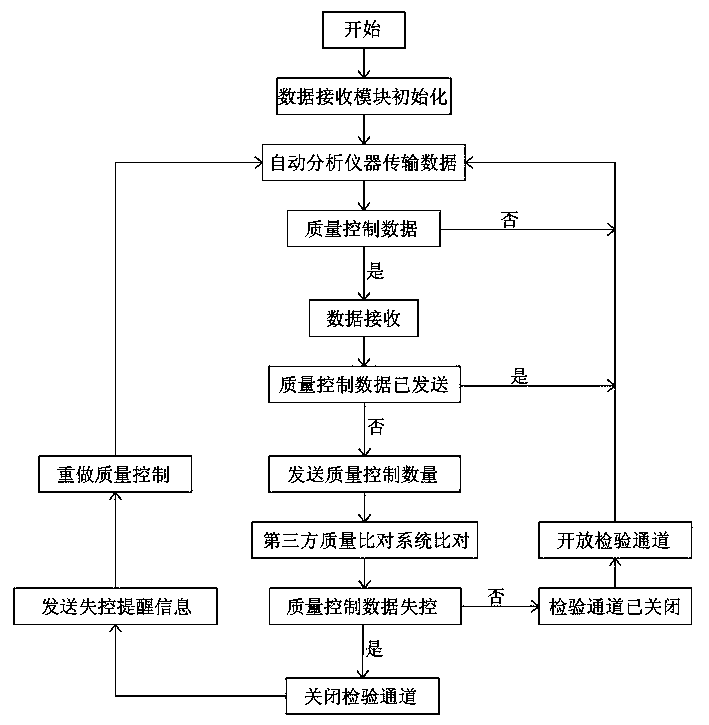
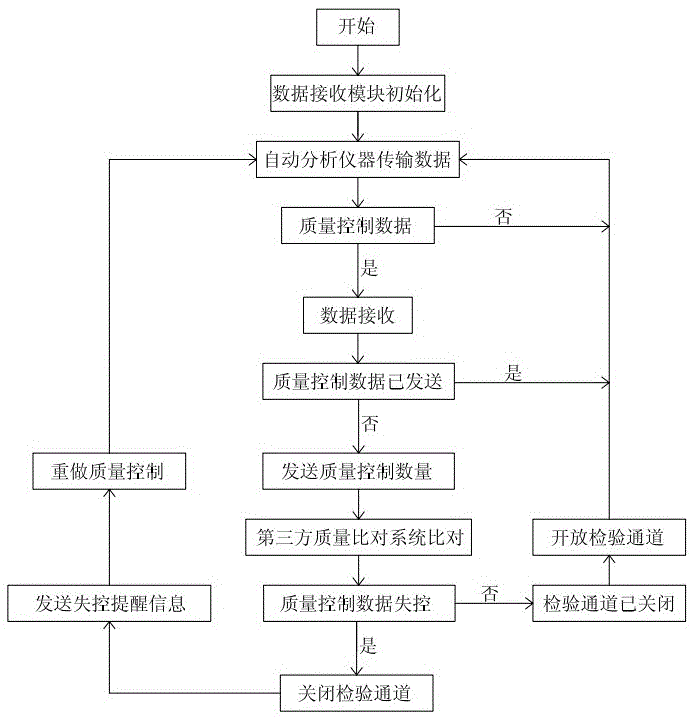
Intelligent control system and intelligent control method for test quality of medical clinical laboratory
InactiveCN103676712AHigh precisionGuaranteed accuracyProgramme control in sequence/logic controllersTest qualityInstrumentation
The invention discloses an intelligent control system for text quality of a medical clinical laboratory. According to quality control data parameters set by the clinical laboratory, the intelligent control system can automatically receive quality control data sent by an automatic analytical instrument of the laboratory, send the quality control data to a professional third-party clinical laboratory test quality comparison system in real time to perform comparison and return comparison results to a laboratory information management system in real time. The invention further discloses an intelligent control method for the test quality of the medical clinical laboratory. By the intelligent control method, real-time data interaction between the laboratory information management system and the third-party clinical laboratory test quality comparison system can be realized without manual intervention, and accordingly, accuracy and reliability of clinical text results are guaranteed.
Owner:JIANGMEN XIANDA COMP TECH CO LTD
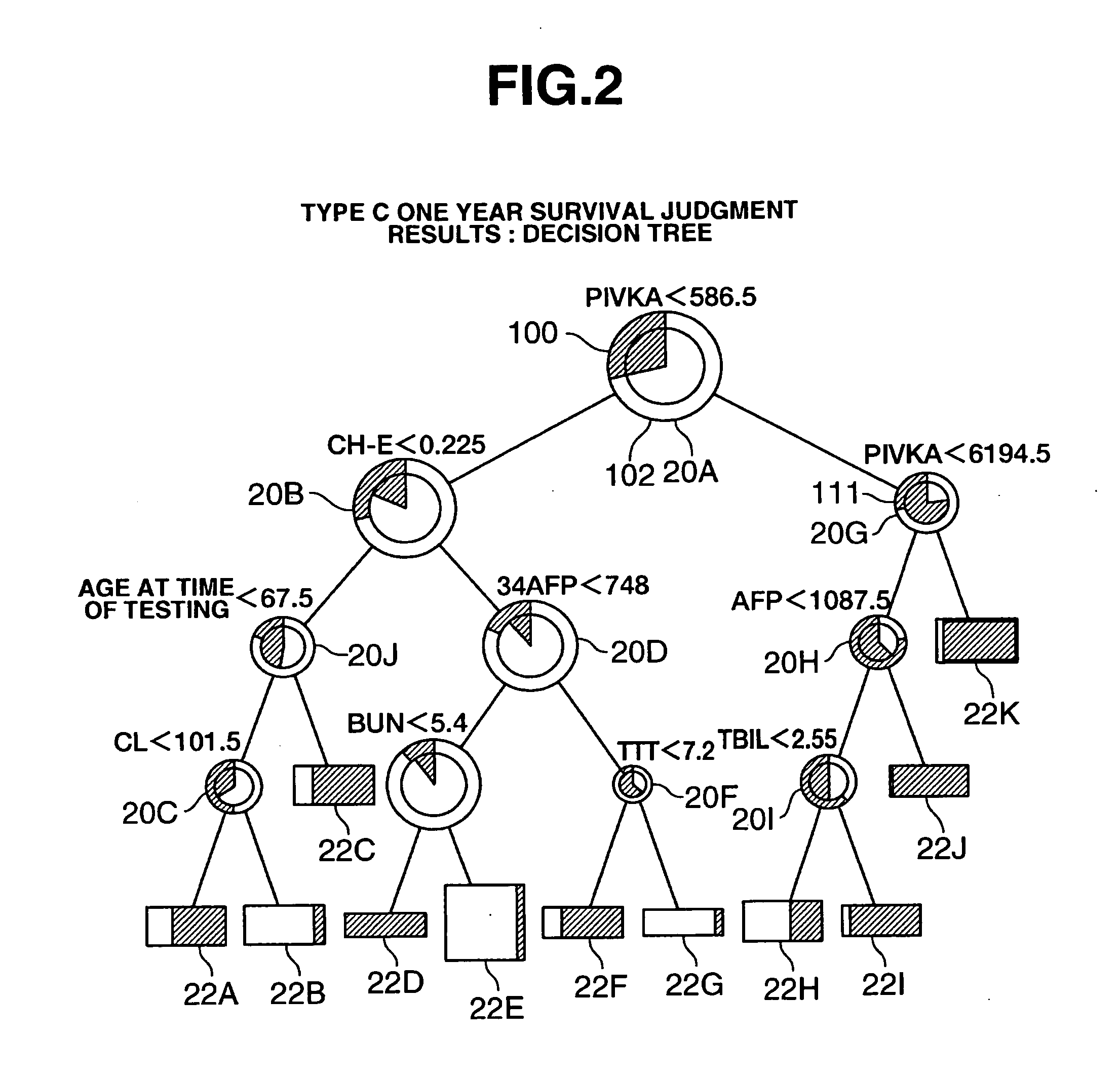
Method of Preparing Disease Prognosis Model, Disease Prognosis Prediction Method using this Model, Prognosis Prediction Device Based on this Model, and Program for Performing the Device and Storage Medium Wherein Said Program is Stored
InactiveUS20070208514A1Add supportAccurate analysisMedical simulationMedical data miningPrognosis predictionDisease cause
The present invention relates to a disease prognosis prediction modeling method for preparing a model for predicting the prognosis of a specified disease from clinical laboratory test values for the disease by means of a computer, the method comprising the steps of: inputting a plurality of actually measured clinical laboratory test values for the disease and actual measured values of the prognoses into the computer; processing these values by a data mining method to determine one or a plurality of clinical laboratory test items which have an influence on the prognosis of the disease; determining a priority of the items with respect to the prognosis in a case where there are a plurality of the items; and establishing a judgment routine in which correlation of the plurality of clinical laboratory test items and the clinical laboratory test value ranges of the test items with the predicted value of the prognosis is stipulated on the basis of the priority, wherein the judgment routine is used as the model.
Owner:EISIA R&D MANAGEMENT CO LTD
Use of superhydrophobic surfaces for liquid agglutination assays
ActiveUS20120264113A1Wide detection rangeFast aggregationBioreactor/fermenter combinationsBiological substance pretreatmentsThermal energyAnalyte
This invention relates to the use of thermodynamically incompatible surfaces in agglutination assays for the express purpose of using the sample as a key component of the detection instrument. Specifically, the invention relates to formation of a lense and a virtual container for rapid mixing via thermal energy by a sample liquid disposed on a superhydrophobic surfaces, and a subsequent specific analyte or overall protein concentration assay using particles agglutination for use in the industrial, environmental, and clinical laboratory test fields.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
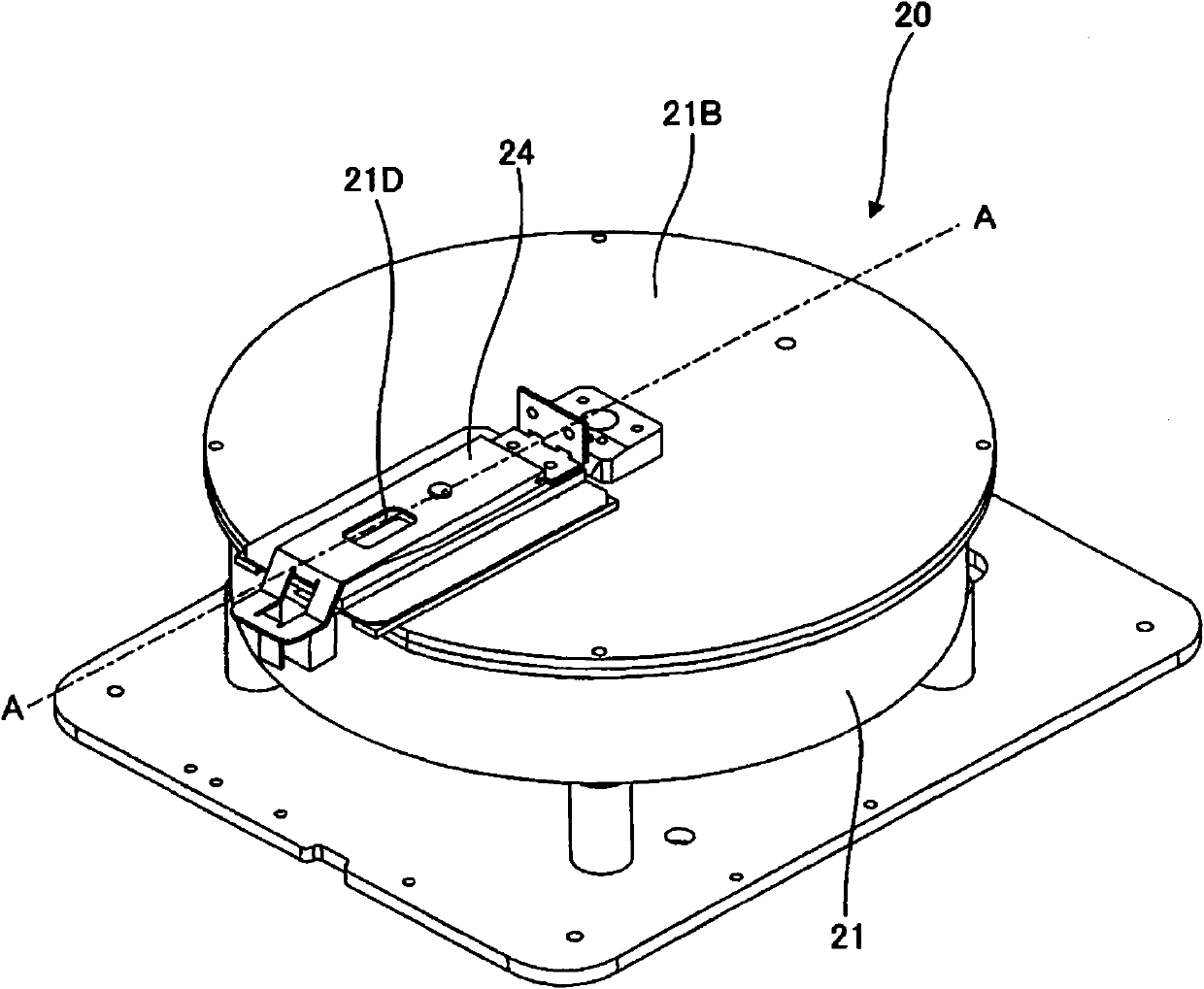
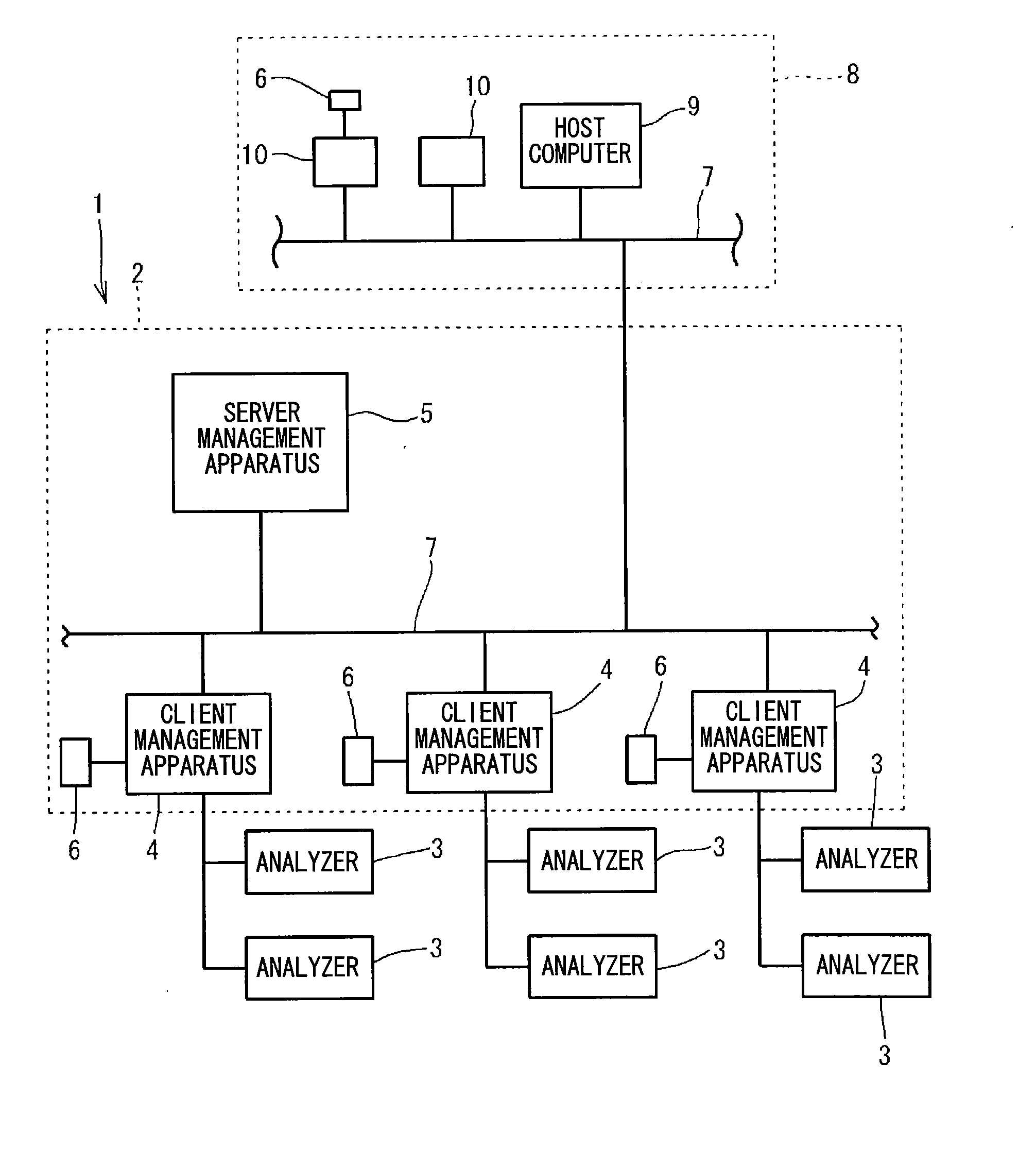
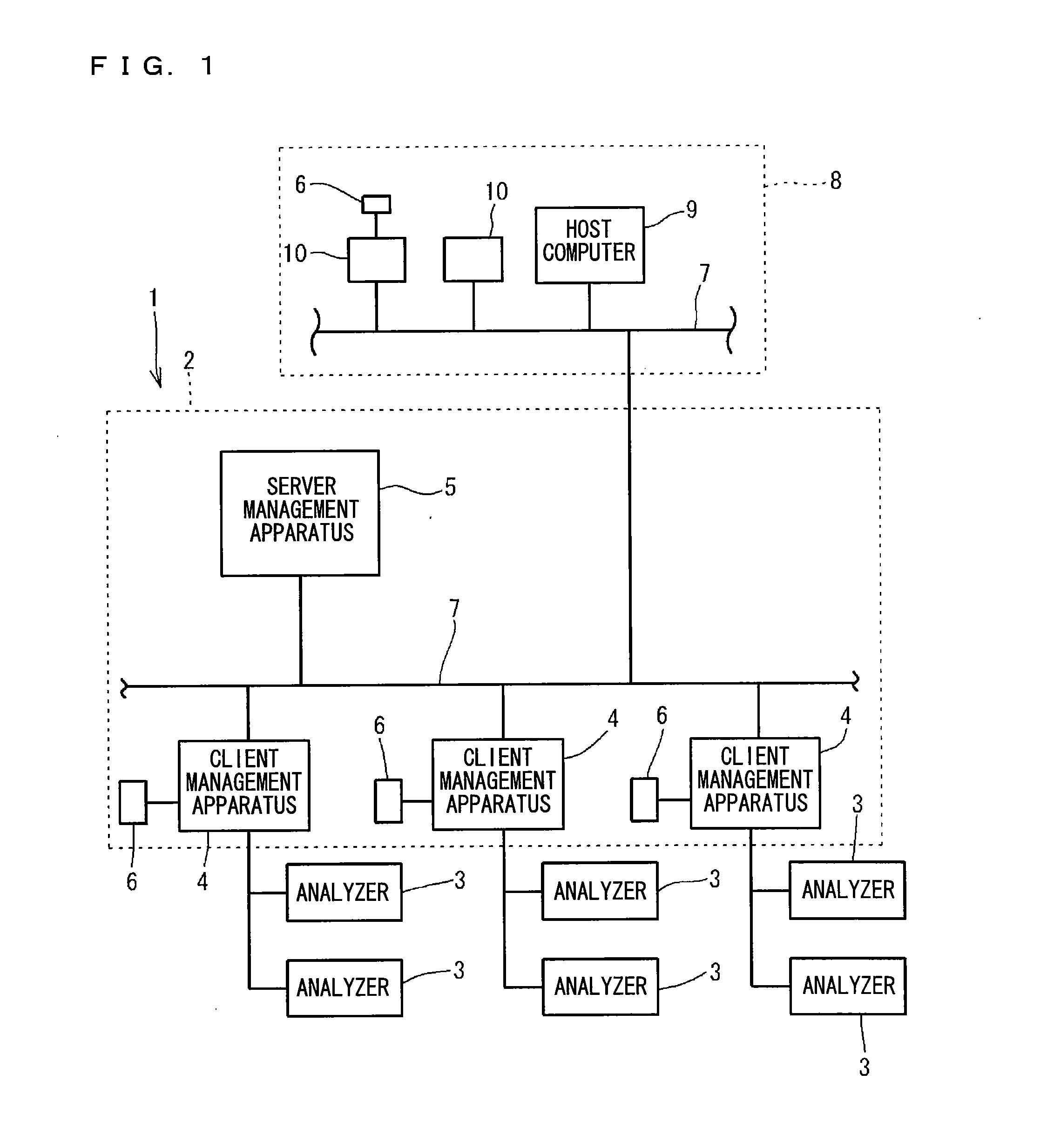
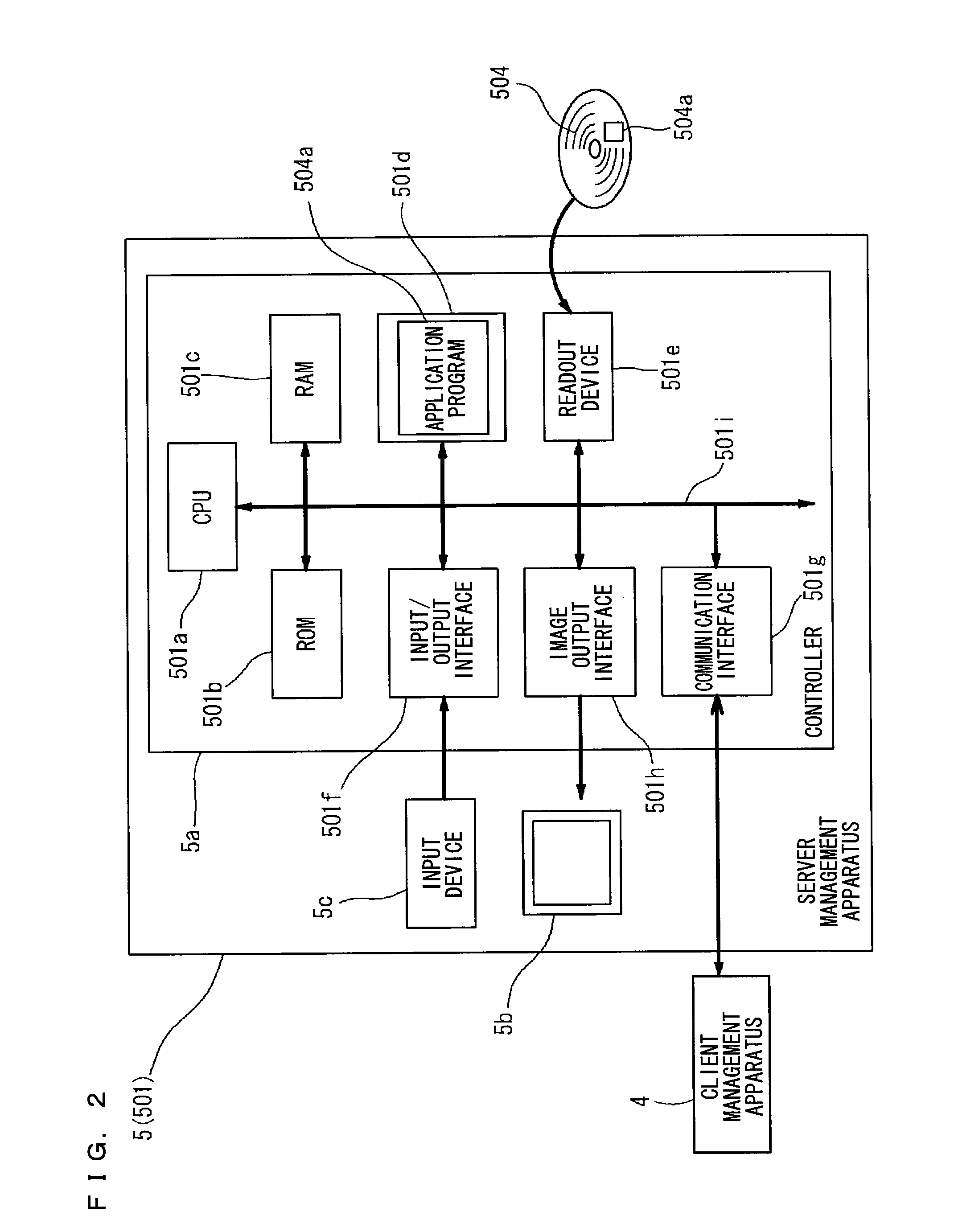
Clinical laboratory test information system
InactiveCN102375929AAppropriate improvementHealthcare resources and facilitiesSpecial data processing applicationsTemporal informationClinical tests
A clinical laboratory test information system comprises: a memory configured to store time information regarding a plurality of stages included in a clinical-laboratory-test-relating operation; an operating device; a display unit; and a controller configured to: cause the display unit to display, based on the time information stored in the memory, first information indicating a temporal transition of a required time for each of the plurality of stages, such that each stage is selectable; and if one of the plurality of stages is selected by the operating device indicated in the first information, cause the display unit to display second information indicating a temporal transition of the required time for the selected stage.
Owner:SYSMEX CORP
Use of superhydrophobic surfaces for liquid agglutination assays
ActiveUS9995688B2Wide detection rangeFast aggregationBioreactor/fermenter combinationsBiological substance pretreatmentsThermal energyAnalyte
This invention relates to the use of thermodynamically incompatible surfaces in agglutination assays for the express purpose of using the sample as a key component of the detection instrument. Specifically, the invention relates to formation of a lense and a virtual container for rapid mixing via thermal energy by a sample liquid disposed on a superhydrophobic surfaces, and a subsequent specific analyte or overall protein concentration assay using particles agglutination for use in the industrial, environmental, and clinical laboratory test fields.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
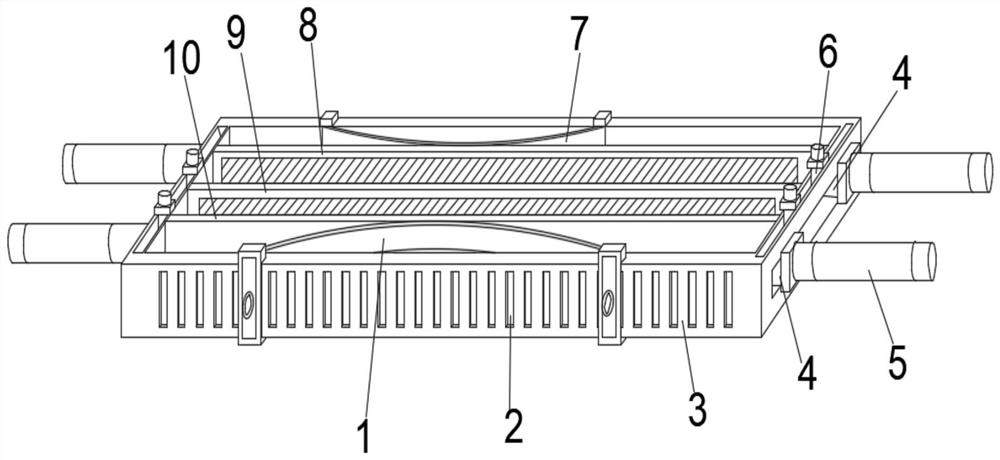
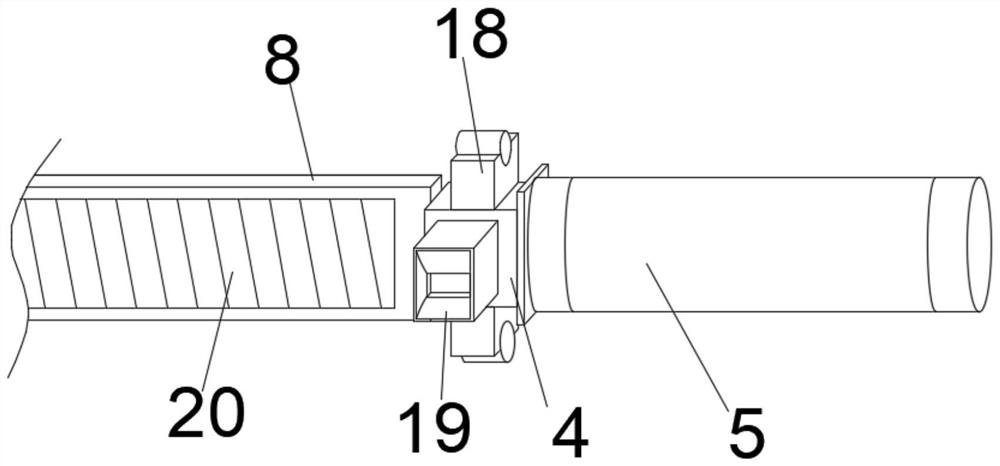
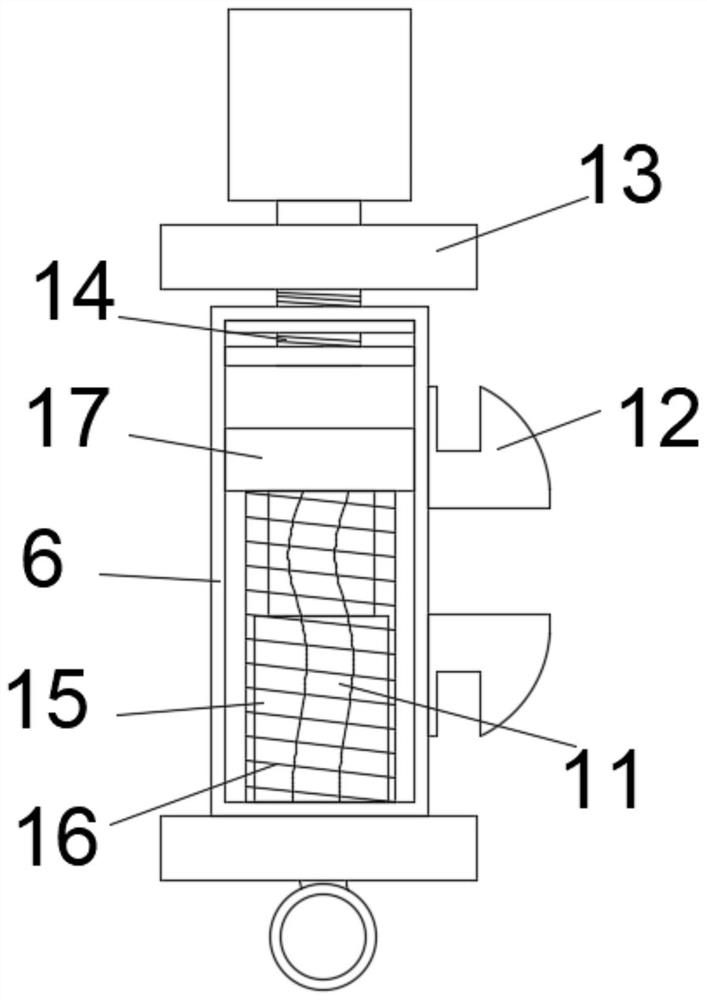
Test tube oscillation device with hydraulic reset function for hospital clinical laboratory
InactiveCN112934081ARealize reciprocating vibrationOscillation effect is uniform and efficientTransportation and packagingMixer accessoriesEngineeringTest tube
The invention discloses a hospital clinical laboratory test tube oscillation device with a hydraulic reset function. The hospital clinical laboratory test tube oscillation device comprises a base, a bracket, an oscillation mechanism, an adjusting mechanism, a test tube and a reset mechanism, wherein the two supports are installed on the front side and the rear side of the top end of the base correspondingly. The oscillating mechanism is assembled at the top end of the base; the adjusting mechanism is assembled on the inner side of the bracket; the test tube is arranged in an inner cavity of the adjusting mechanism; and the reset mechanism is assembled at the top of the test tube. The device can simultaneously oscillate a plurality of test tubes with different or same calibers at one time, is higher in practicability, replaces a manual oscillation mode, realizes all-directional reciprocating oscillation of liquid in inner cavities of the test tubes from all angles, is more uniform and efficient in oscillation effect, and is higher in practicability. Besides, the device can balance the pressure intensity of the inner cavity of the test tube after oscillation, so that the normal resetting of liquid in the inner cavity of the test tube is effectively ensured, and the use requirements are satisfied well.
Owner:JINAN THE THIRD HOSPITAL
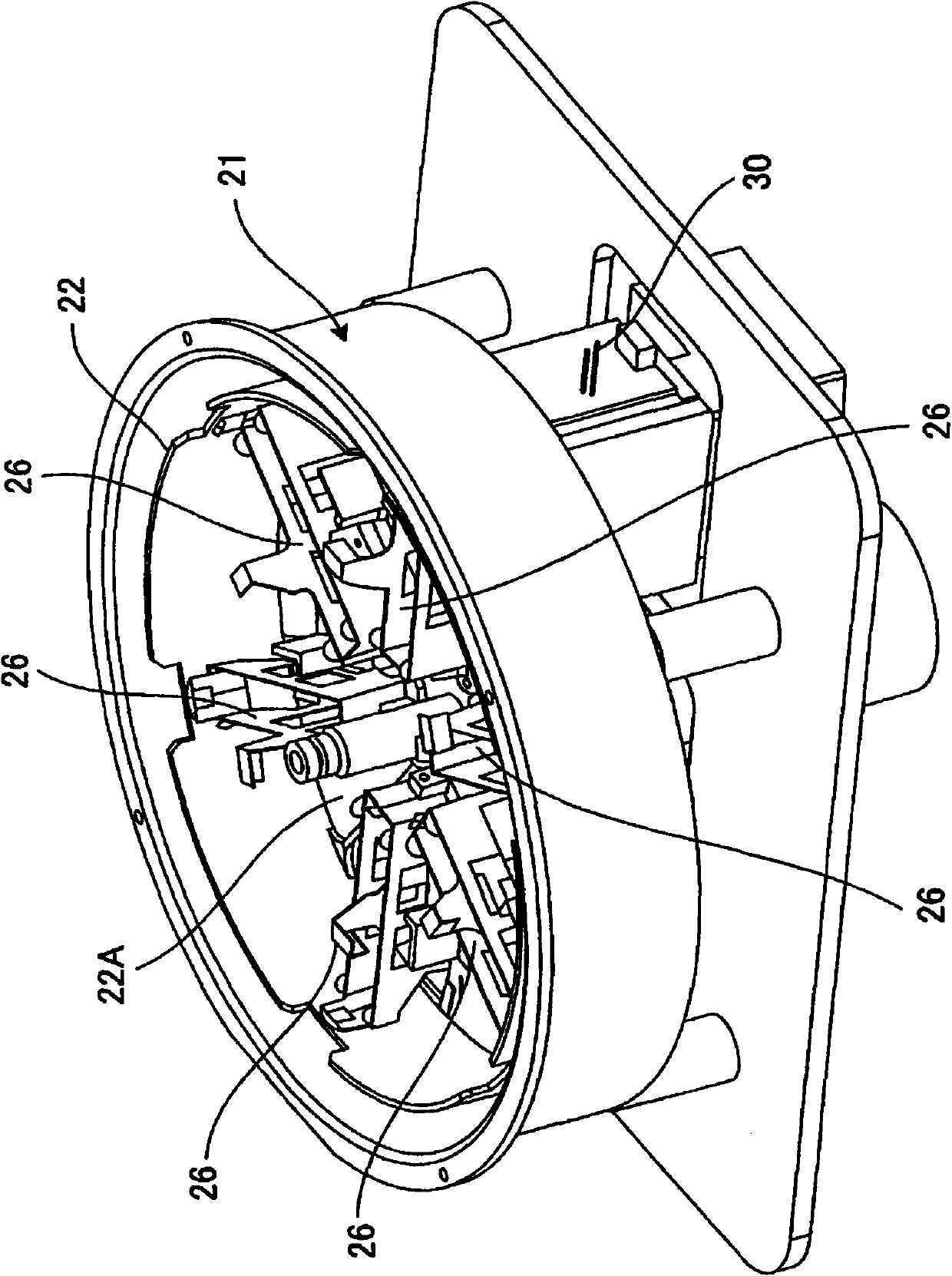
Clinical laboratory test information system and non-transitory storage medium
InactiveUS20120041776A1Data processing applicationsHospital data managementTemporal informationComputer science
A clinical laboratory test information system comprising: a memory configured to store time information regarding a plurality of stages included in a clinical-laboratory-test-relating operation; an operating device; a display unit; and a controller configured to: cause the display unit to display, based on the time information stored in the memory, first information indicating a temporal transition of a required time for each of the plurality of stages, such that each stage is selectable; and if one of the plurality of stages is selected by the operating device indicated in the first information, cause the display unit to display second information indicating a temporal transition of the required time for the selected stage. Also, a non-transitory storage medium.
Owner:SYSMEX CORP
Performing data analysis on clinical data
ActiveUS9639667B2Medical data miningComputer-assisted medical data acquisitionDatum referenceStatistical analysis
Owner:ALBANY MEDICAL COLLEGE
High-stability clinical laboratory test tube clamp
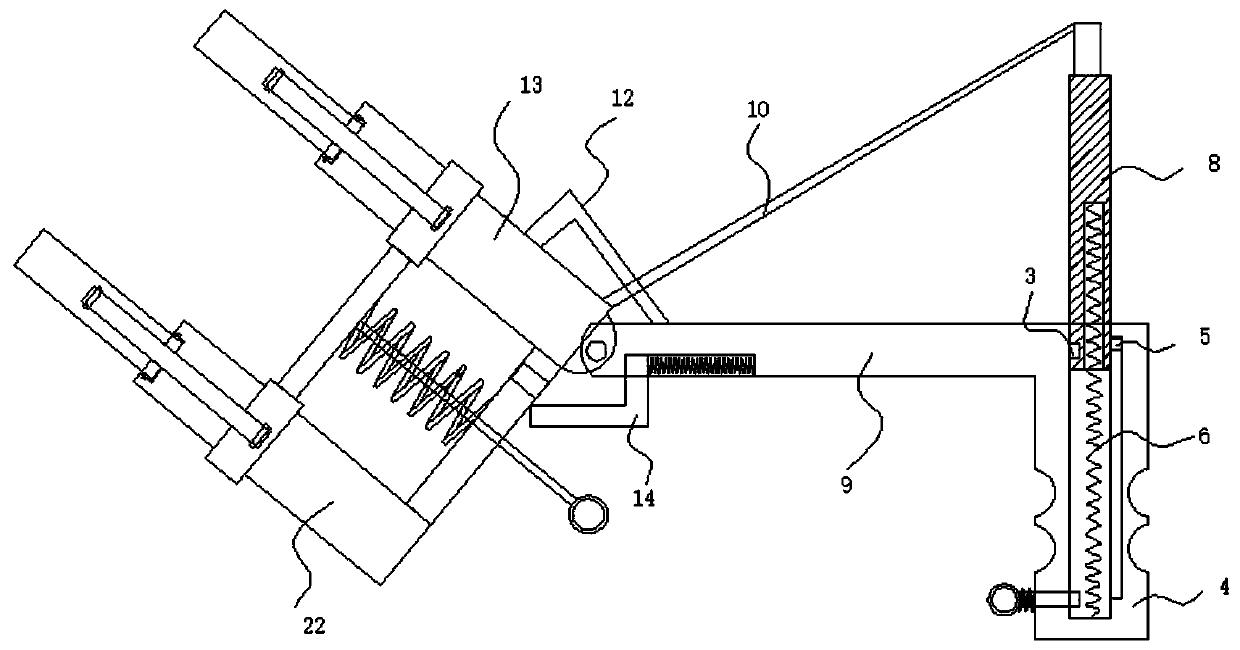
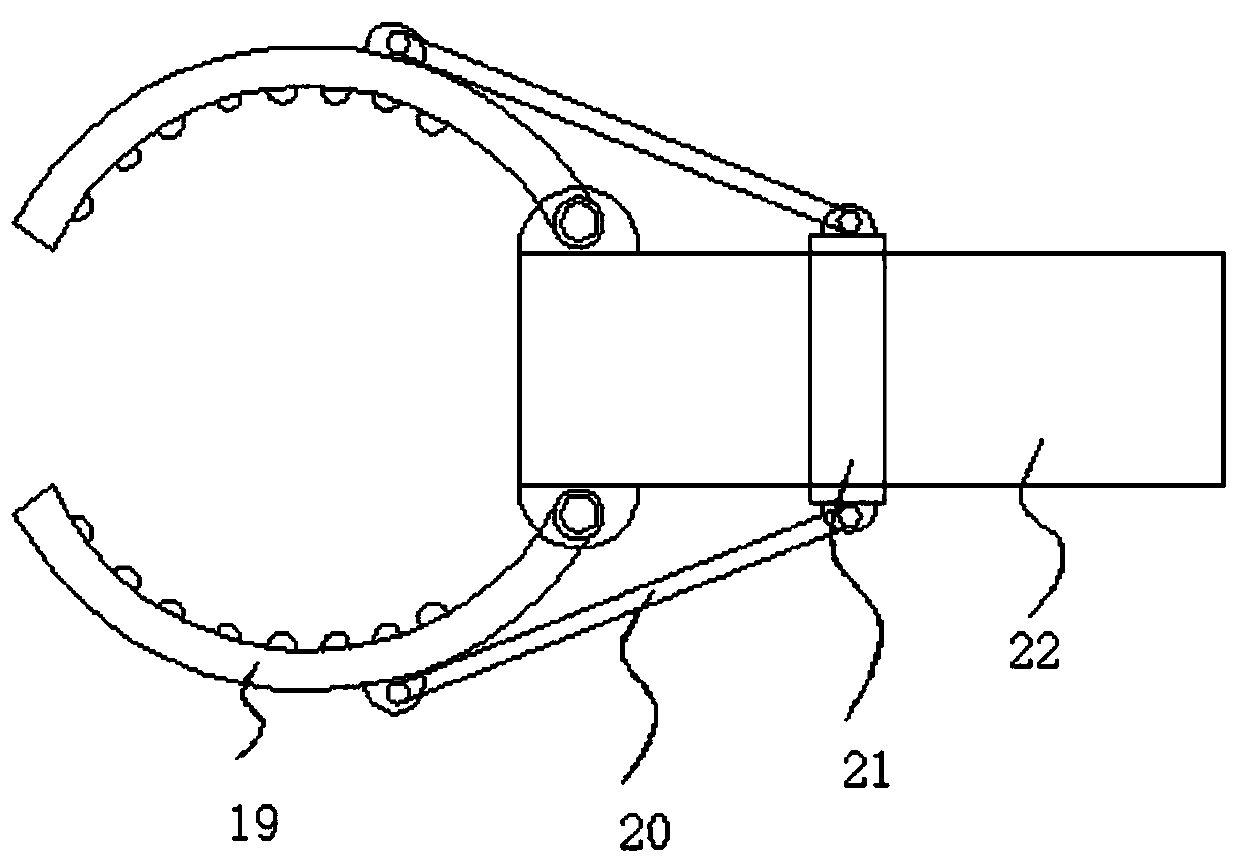
InactiveCN111468209ANot easy to fall offImprove stabilityTest tube stands/holdersEngineeringStructural engineering
The invention belongs to the field of test tube clamps, and particularly provides a high-stability clinical laboratory test tube clamp which comprises a handle, a holding column, a first fixing columnand a second fixing column; one end of the handle is connected with the holding column, the other end of the handle is hinged to the first fixing column, and a bending power mechanism is arranged between the holding column and the first fixing column; the bottom end of the first fixing column is fixedly connected with a second fixing column through a connecting column, the other end of the firstfixing column and the other end of the second fixing column are each hinged to two symmetrically-arranged clamping jaws, the clamping jaws are each hinged to an action rod, the first fixing column andthe second fixing column are each sleeved with a movable ring in a sliding mode, and the movable rings are hinged to two action rods located on the same horizontal plane. According to the invention,two parts of a test tube can be clamped and fixed, the stability is good, the test tube is not prone to falling off in the operation process, the two clamping parts can be driven to move at the same time through pulling of one pull rod to achieve rapid clamping, and operation is convenient and rapid.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Multifunctional experiment platform for clinical inspection
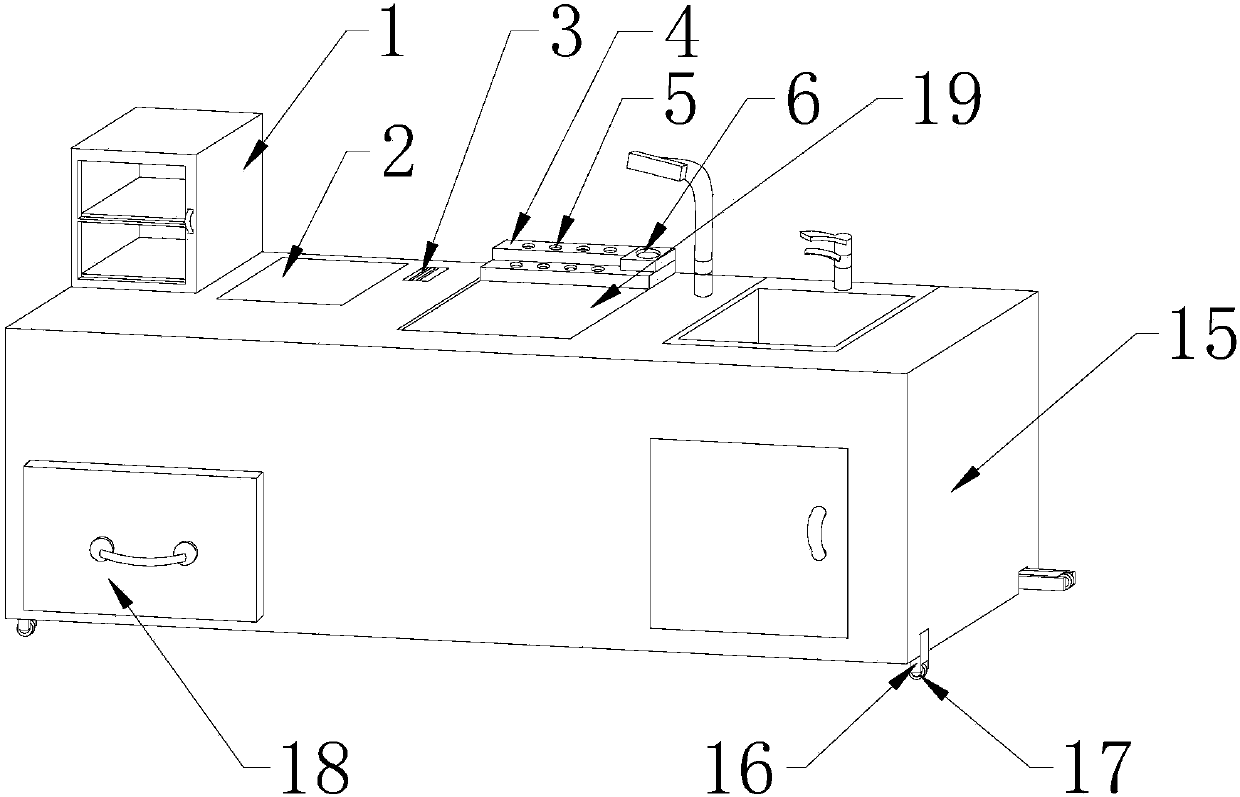
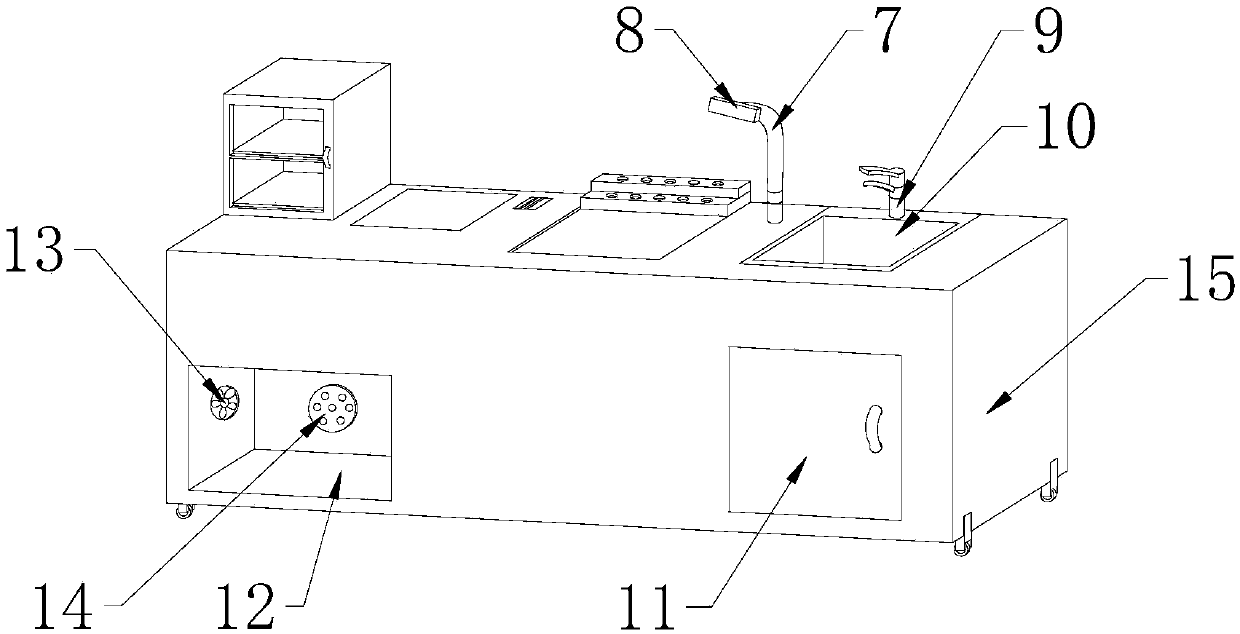
InactiveCN107661786ASolve the problem of monotonous designAvoid breedingLaboratory benches/tablesEngineeringLaboratory facility
The invention discloses a multifunctional experimental platform for clinical examination, which comprises a platform body, a disinfection cabinet is fixed at the left end of the top of the platform body, and the disinfection cabinet is in the shape of a "cuboid", and a control touch screen is provided at the right end of the disinfection cabinet, and the control touch screen The screen is embedded in the platform body, a workbench is arranged in the middle of the top of the platform body, and the workbench is embedded in the platform body, and a reagent rack is arranged at the rear end of the workbench. This kind of multifunctional experimental platform for clinical examination is equipped with a zigzag rod and a pulley. The bottom of the zigzag rod is provided with a pulley, and the pulley is movably connected with the zigzag rod. It is bent at ten degrees, and the bottom of the bending rod is equipped with a pulley, so that the platform body can slide and move, which is convenient for handling and solves the problem of troublesome handling of the platform body in the past, wasting manpower and time, and is suitable for multifunctional experimental platforms for clinical testing. Production and use have good development prospects.
Owner:郑林娜
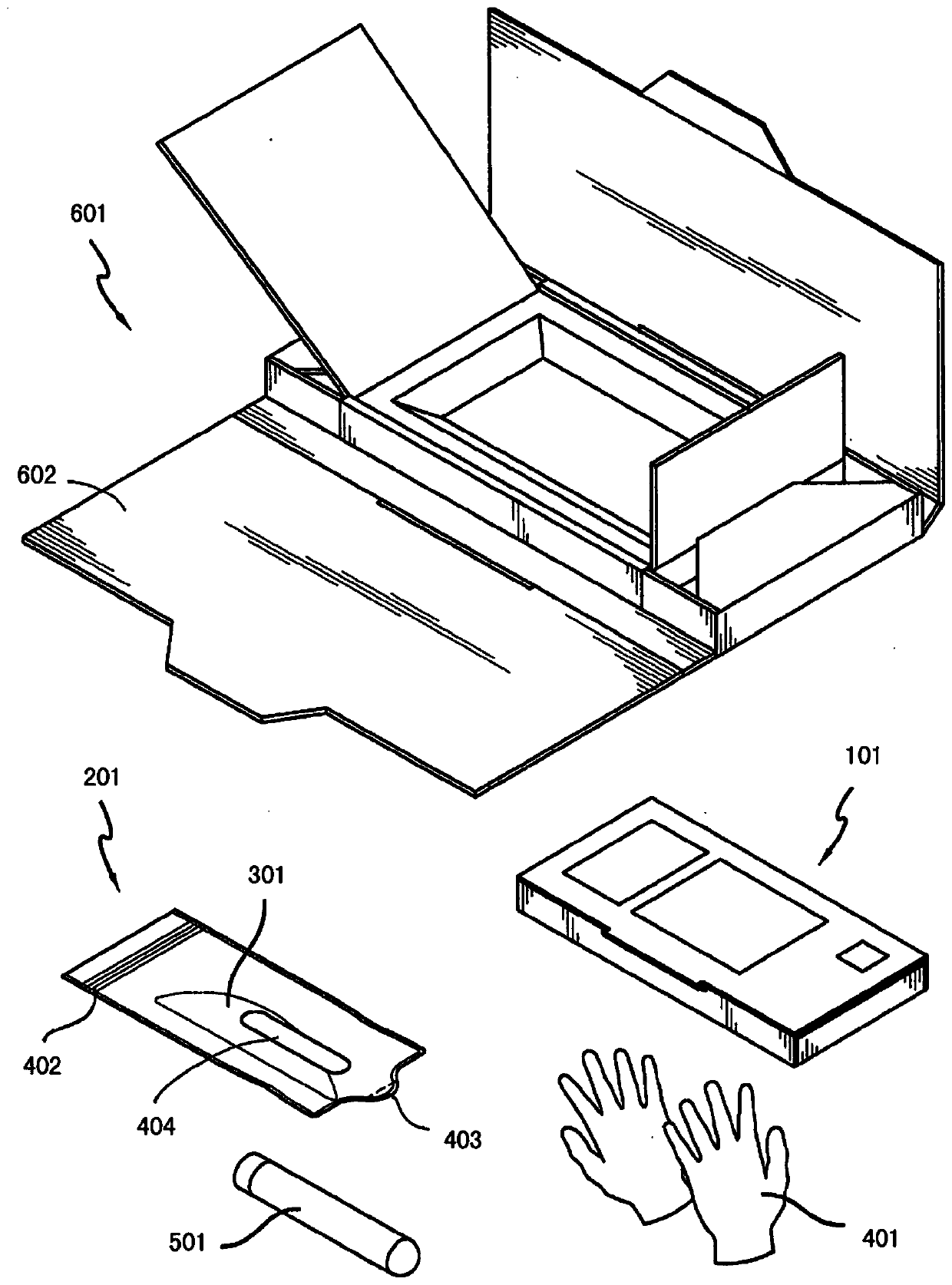
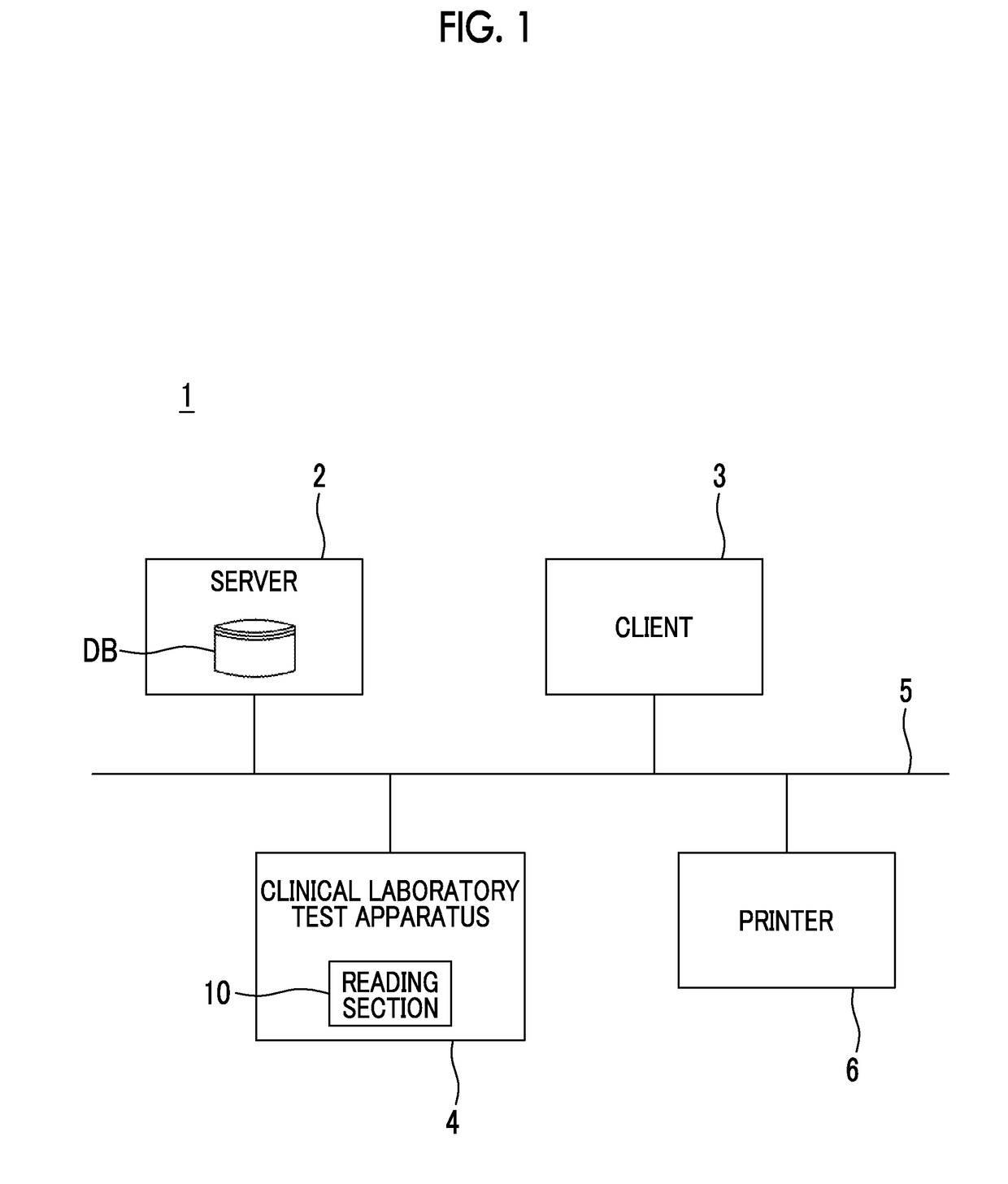
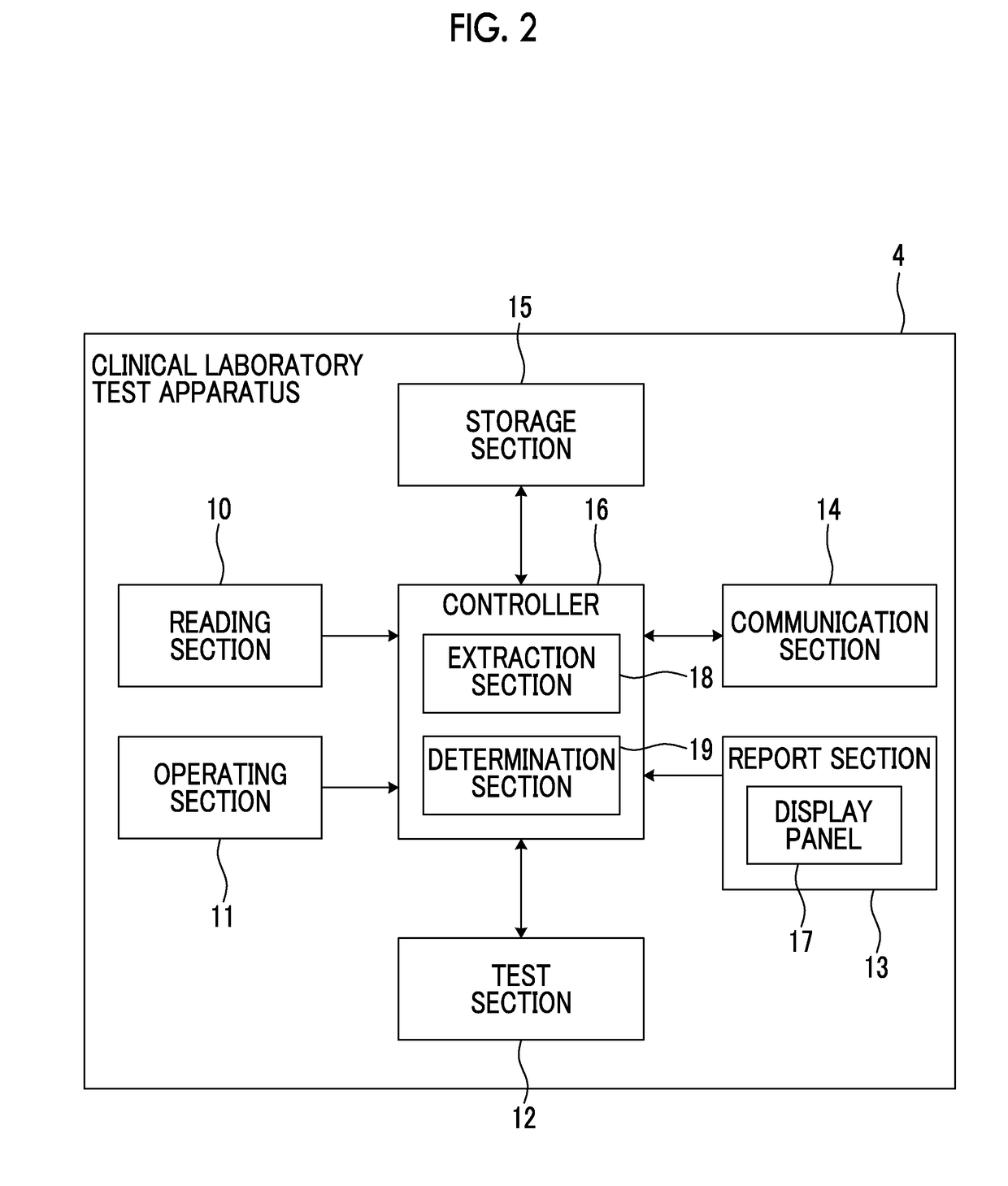
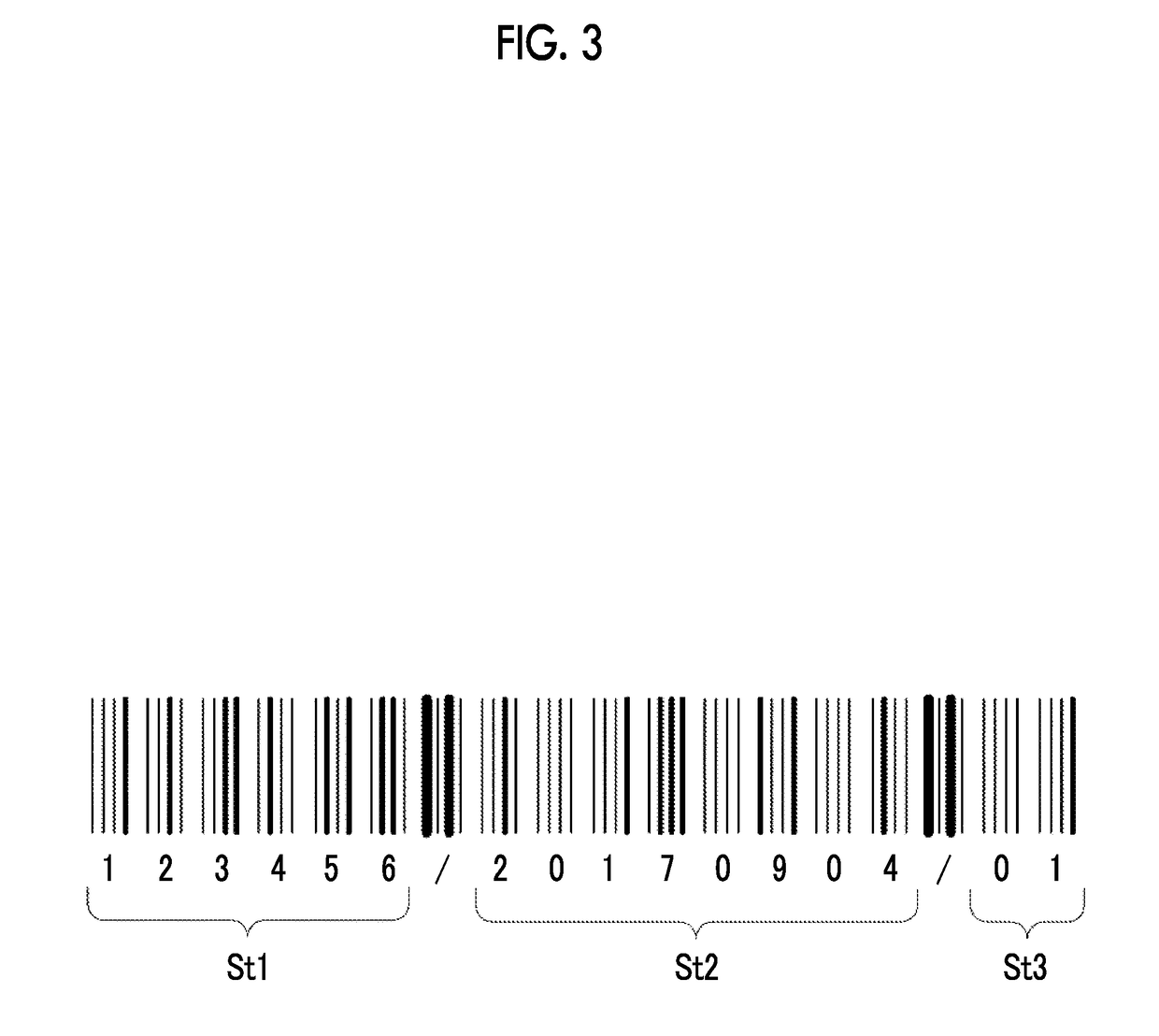
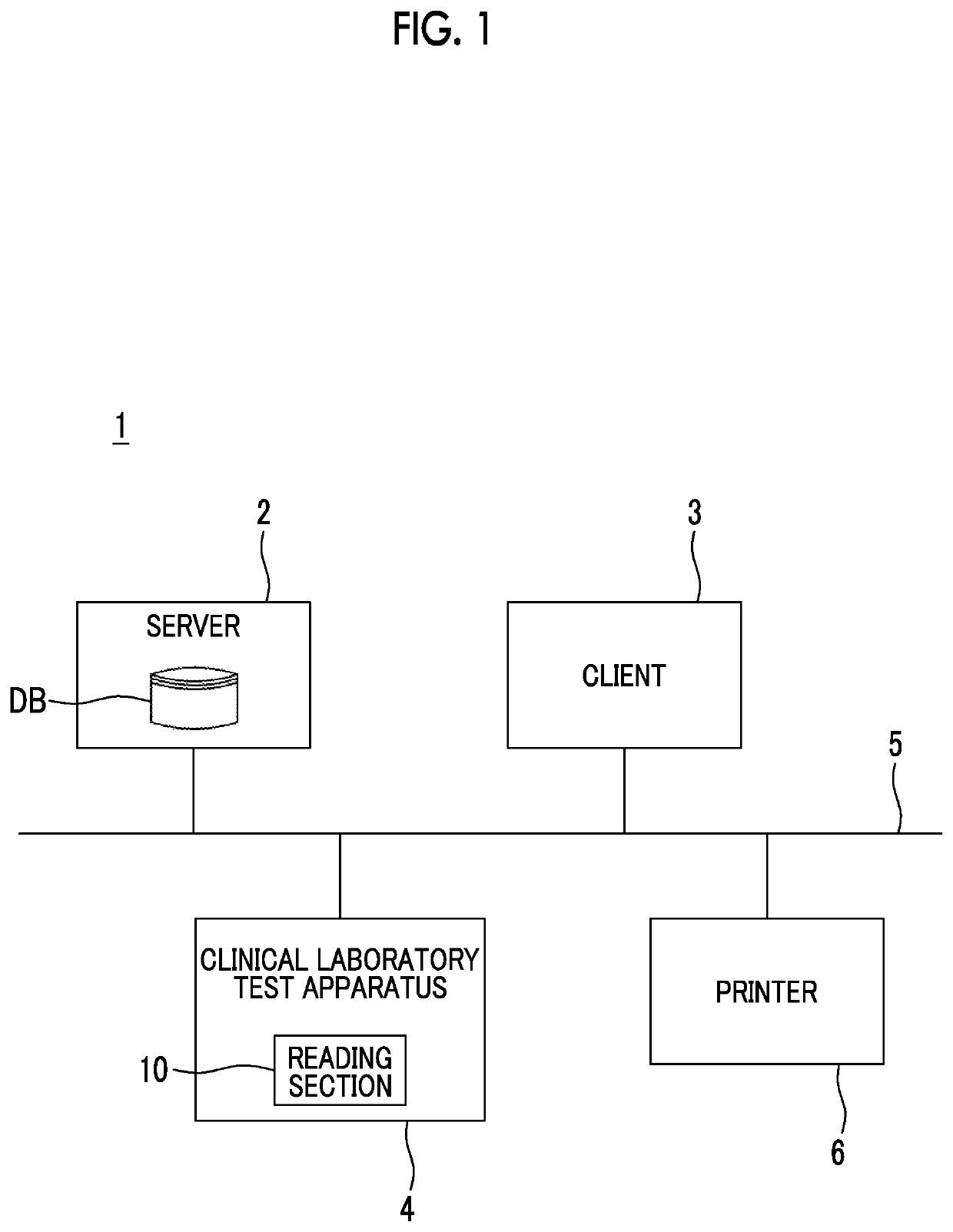
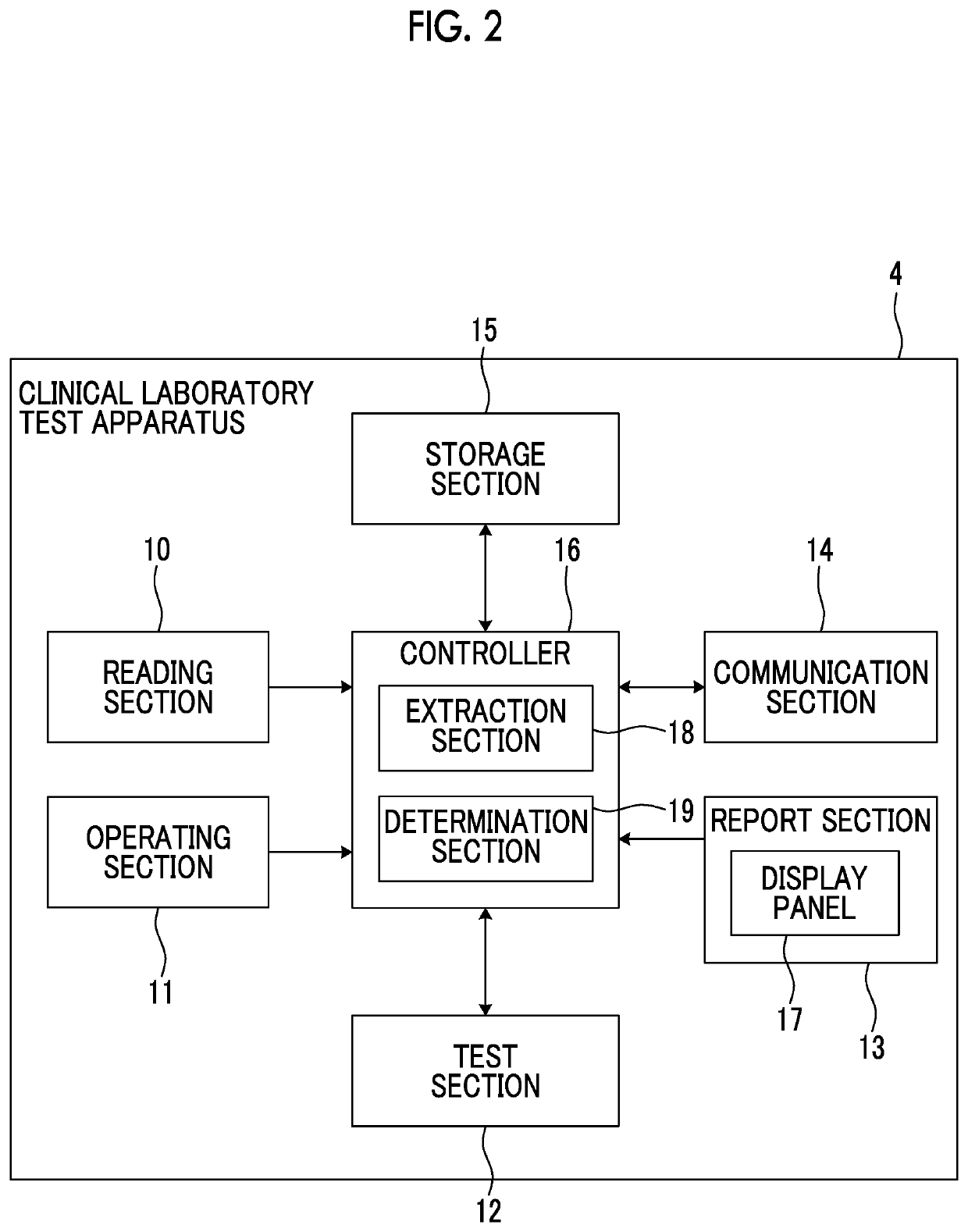
Clinical laboratory test apparatus and system
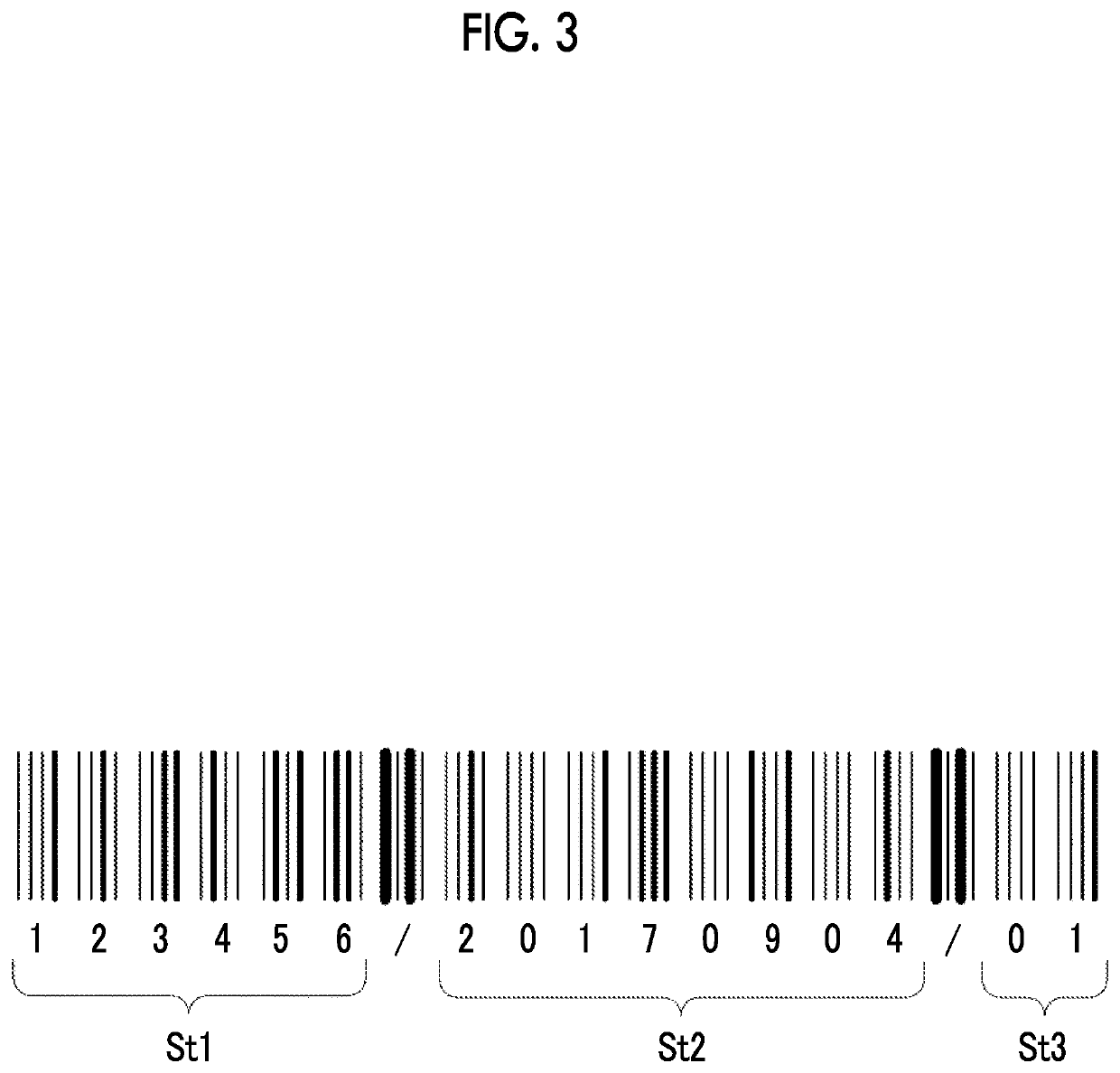
ActiveUS20190080786A1Guaranteed uptimeSmooth managementLaboratory glasswaresRecord carriers used with machinesComputer scienceLaboratory facility
A clinical laboratory test apparatus of a health care information system includes a reading section that reads an optically readable code array, an extraction section that extracts, from a plurality of the code arrays read by the reading section, a rule that is common between the plurality of code arrays, a storage section that stores the rule extracted by the extraction section, and a determination section that determines authenticity of each of the code arrays read by the reading section based on the rule stored in the storage section. In testing a sample, in a case where it is determined by the determination section that the code array read by the reading section is true, the test is executed, and in a case where it is determined that the code array is false, the execution of the test is suspended.
Owner:FUJIFILM CORP
An intelligent control system and an intelligent control method for the inspection quality of a medical clinical laboratory
InactiveCN103676712BGuaranteed accuracyGuaranteed reliabilityProgramme control in sequence/logic controllersTest qualityInstrumentation
The invention discloses an intelligent control system for text quality of a medical clinical laboratory. According to quality control data parameters set by the clinical laboratory, the intelligent control system can automatically receive quality control data sent by an automatic analytical instrument of the laboratory, send the quality control data to a professional third-party clinical laboratory test quality comparison system in real time to perform comparison and return comparison results to a laboratory information management system in real time. The invention further discloses an intelligent control method for the test quality of the medical clinical laboratory. By the intelligent control method, real-time data interaction between the laboratory information management system and the third-party clinical laboratory test quality comparison system can be realized without manual intervention, and accordingly, accuracy and reliability of clinical text results are guaranteed.
Owner:JIANGMEN XIANDA COMP TECH CO LTD
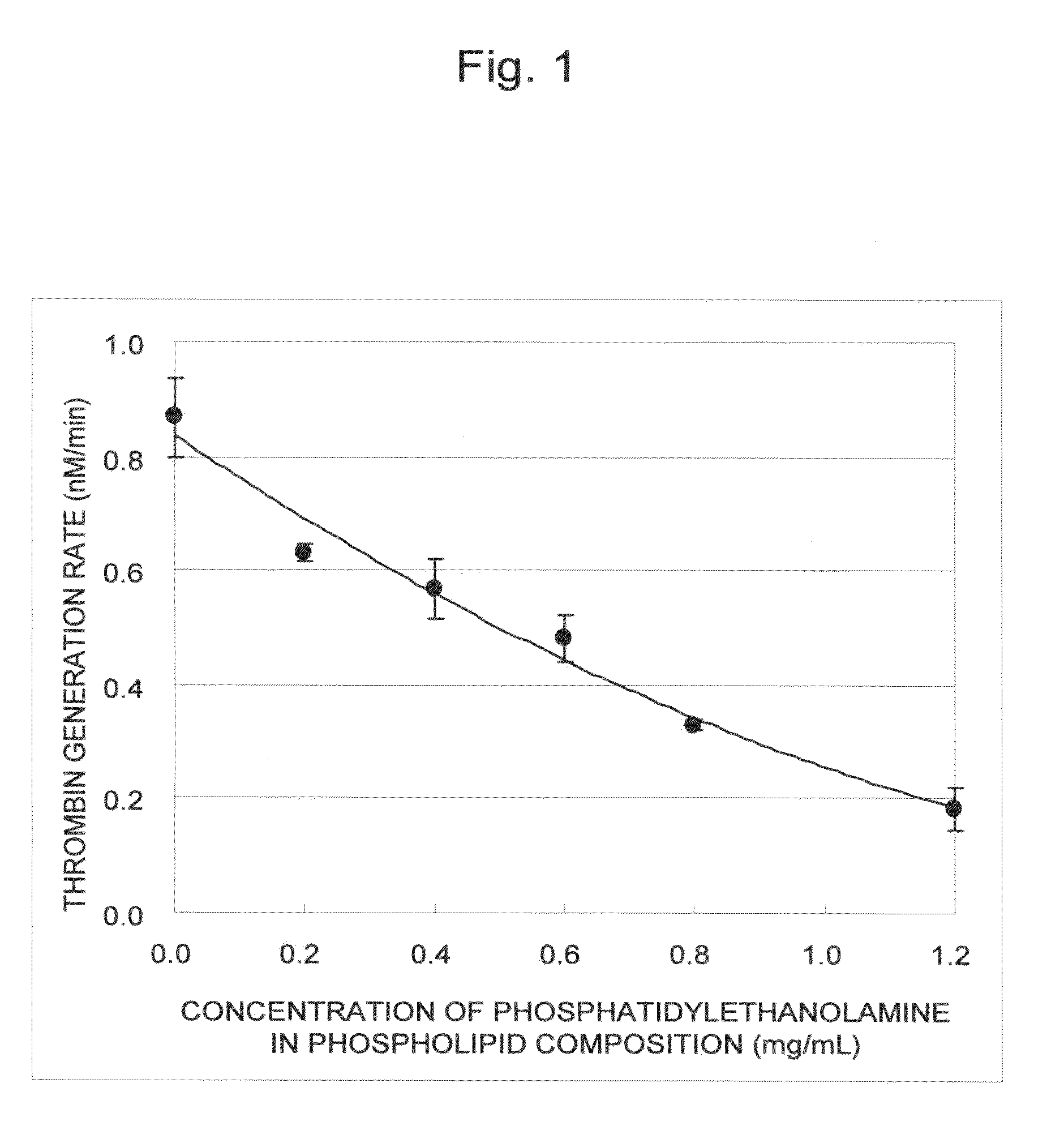
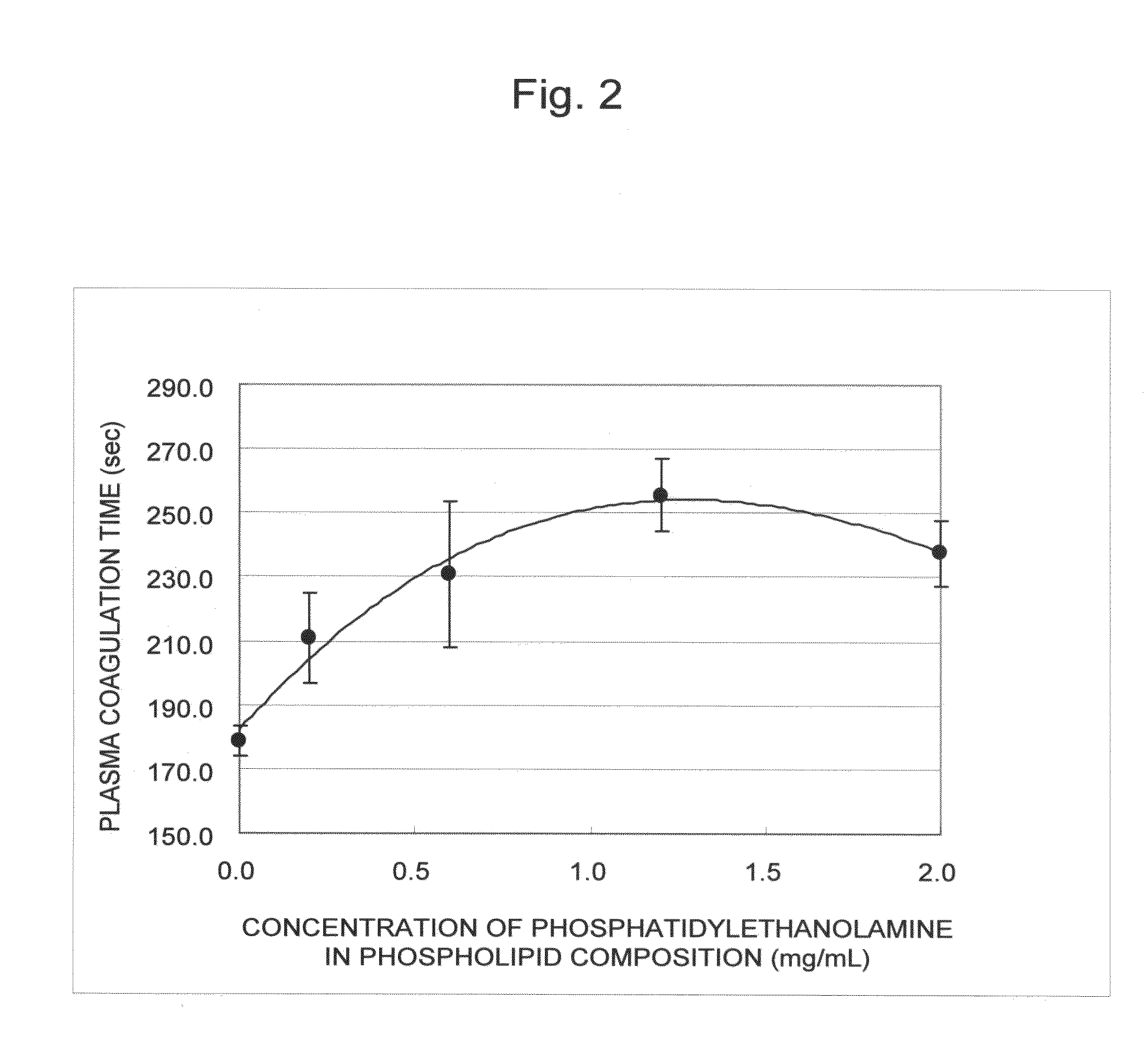
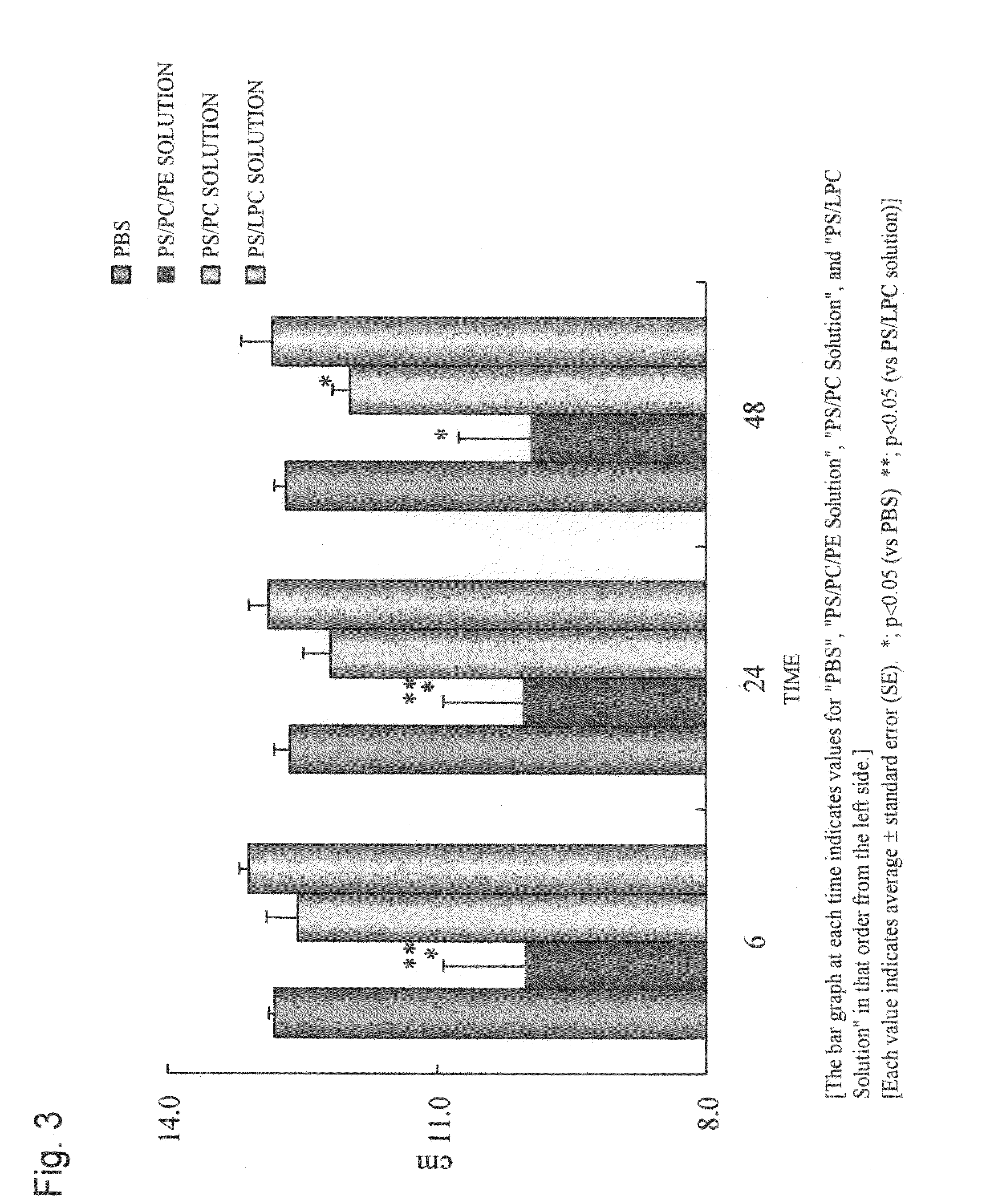
Blood coagulation reaction inhibitor
InactiveUS20120129808A1Prevent thrombosisGood coagulationBiocideOrganic active ingredientsAntithrombotic AgentThrombus
The present invention is a blood coagulation reaction inhibitor comprising phosphatidylserine, phosphatidylcholine, and phosphatidylethanolamine. The present invention is also a thrombin generation inhibitor comprising phosphatidylserine, phosphatidylcholine, and phosphatidylethanolamine. The blood coagulation reaction inhibitor and the thrombin generation inhibitor of the present invention are useful as an antithrombotic agent, a clinical laboratory test reagent for the blood coagulation-fibrinolysis system, and the like.
Clinical laboratory test apparatus and system
ActiveUS10497468B2Smooth managementGuaranteed uptimeLaboratory glasswaresRecord carriers used with machinesTest sampleComputer science
A clinical laboratory test apparatus of a health care information system includes a reading section that reads an optically readable code array, an extraction section that extracts, from a plurality of the code arrays read by the reading section, a rule that is common between the plurality of code arrays, a storage section that stores the rule extracted by the extraction section, and a determination section that determines authenticity of each of the code arrays read by the reading section based on the rule stored in the storage section. In testing a sample, in a case where it is determined by the determination section that the code array read by the reading section is true, the test is executed, and in a case where it is determined that the code array is false, the execution of the test is suspended.
Owner:FUJIFILM CORP
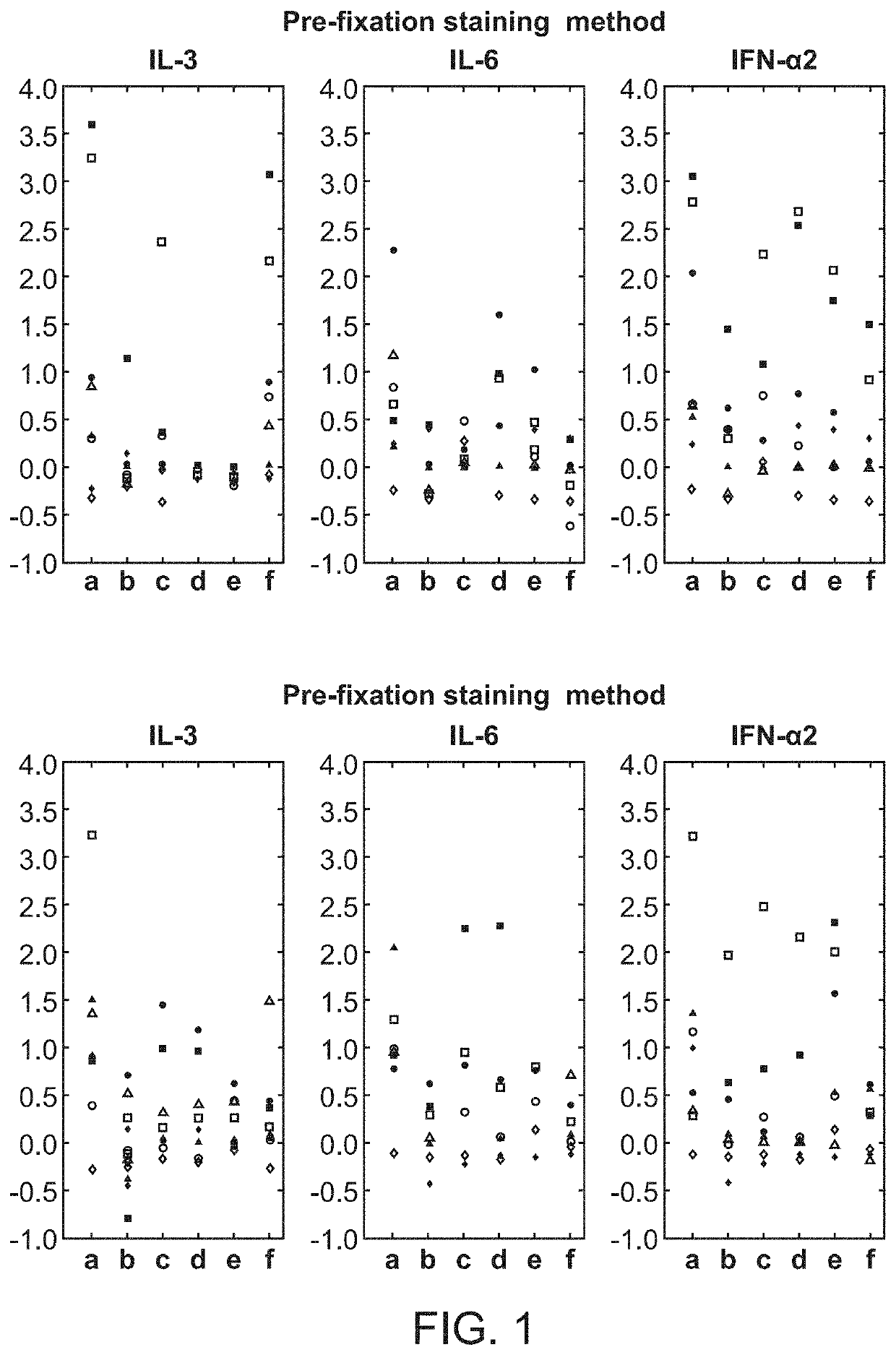
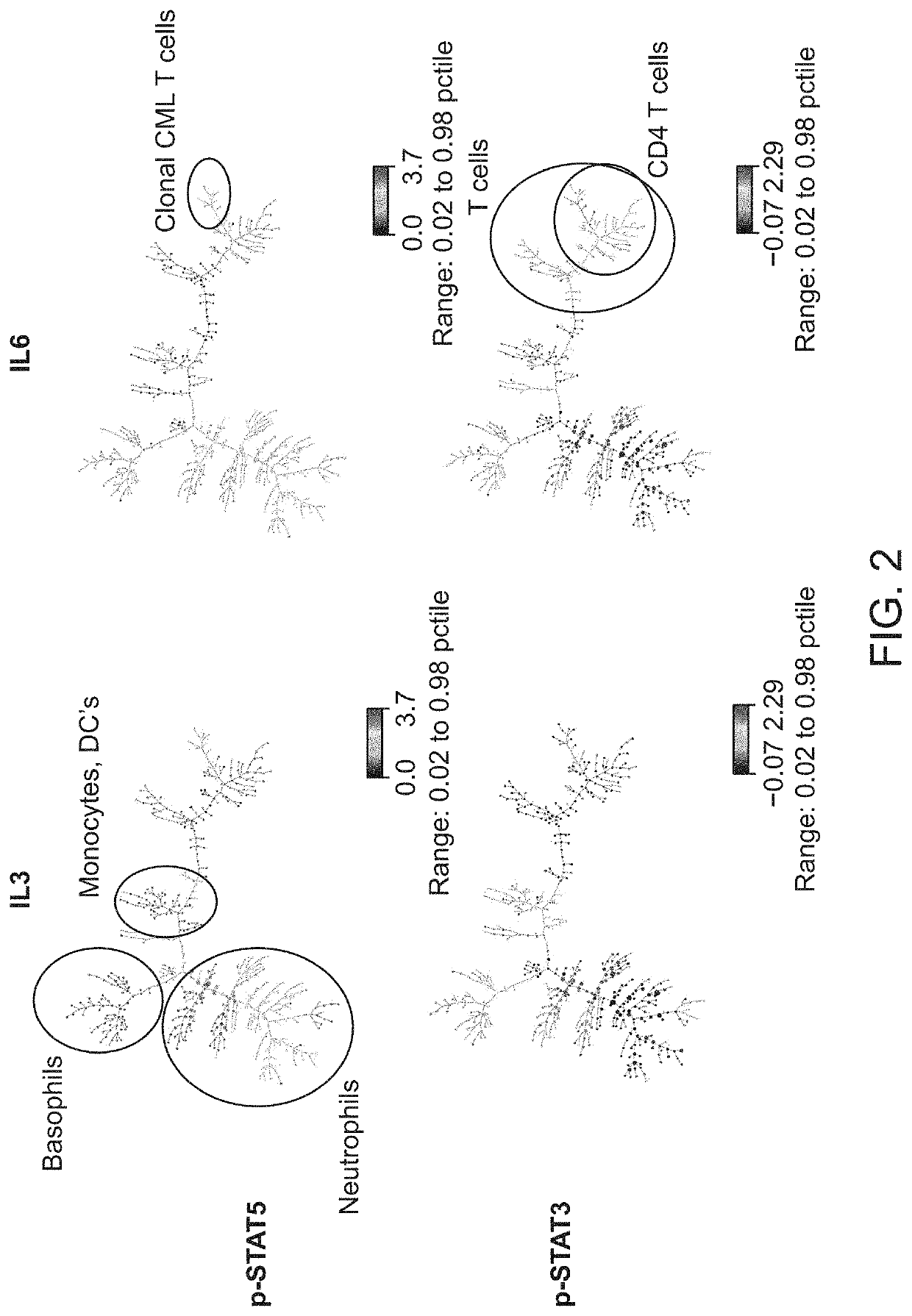
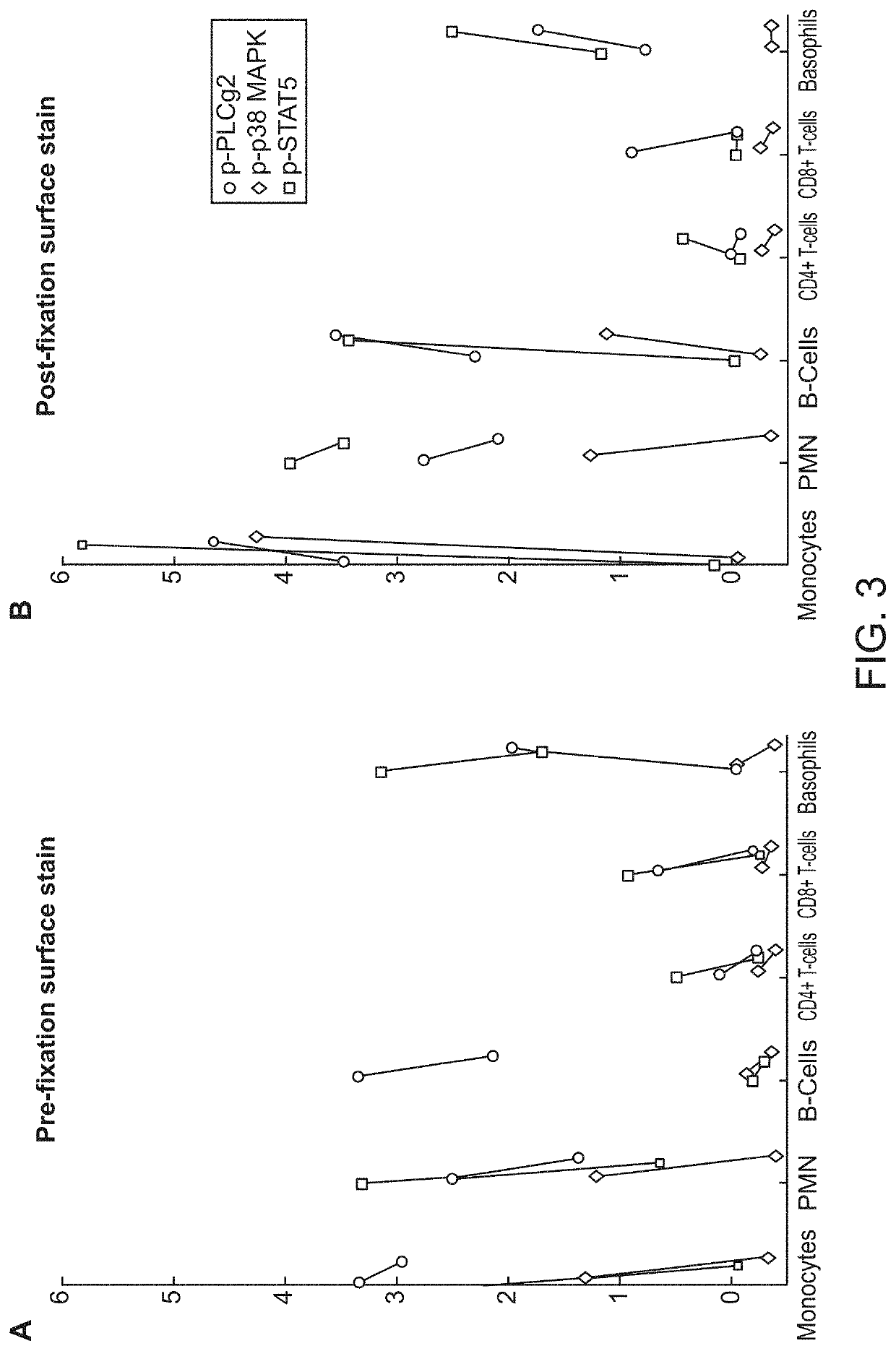
Cell-specific signaling biomarker analysis by high parameter cytometry; sample processing, assay set-up, method, analysis
ActiveUS10845363B2Fine surfaceHigh activityDisease diagnosisIndividual particle analysisDiagnosis laboratoryMarker selection
The present invention recognizes that current clinical laboratory testing methods for multiparametric single cell analysis are limited to analysis of intact live cells, and are insufficient for identification of signaling activation profile defining certain cell types, including but not limited to neoplastic and immunologically activated cell subsets. One aspect of the present invention generally relates to marker selection in panels to include proteins routinely assessed in standard FCM, while preferably also incorporating markers for surface receptor proteins within activated signaling cascades. A further aspect of the present invention generally relates to panel design for the following indications in neoplastic and non-neoplastic clinical applications as examples of the technology: (a) identification of CML progenitor cell subsets in the setting of disease recurrence after treatment discontinuation or relapse due to treatment resistance, and (b) characterization of activated basophils to predict the severity of an allergic response. Another aspect of the present invention generally relates to methods to measure levels of surface and IC biomarkers in separate or combined assays for robust characterization of each or select cell compartment, and data analysis based on results from each or all method(s) used for optimal detection of the markers. A further aspect of the present invention generally relates to the identification and profiling of cell subpopulations based on analysis of surface markers including those associated with lineage and maturation of cell types and receptor proteins, and the corresponding IC phosphoproteins including those in activated signaling cascades to predict certain disease states or response to treatment.
Owner:DEEPATH MEDICAL
Clinical laboratory test tube storage device with an anti-collision function
InactiveCN113042133AAvoid shakingGood anti-collision performanceTest tube stands/holdersEngineeringTop cap
The invention discloses a clinical laboratory test tube storage device with an anti-collision function. The clinical laboratory test tube storage device comprises a test tube rack, a top cover is arranged at the top end of the test tube rack, a loading groove is formed in the top end of the test tube rack, a ventilation mechanism is arranged in an inner cavity of the top cover, and a clamping mechanism is arranged in an inner cavity of the test tube rack. Through the ventilation mechanism, sealed test tubes can be selectively ventilated or sealed, and corresponding operation treatment can be carried out according to different test tube containing components.
Owner:JINAN THE THIRD HOSPITAL
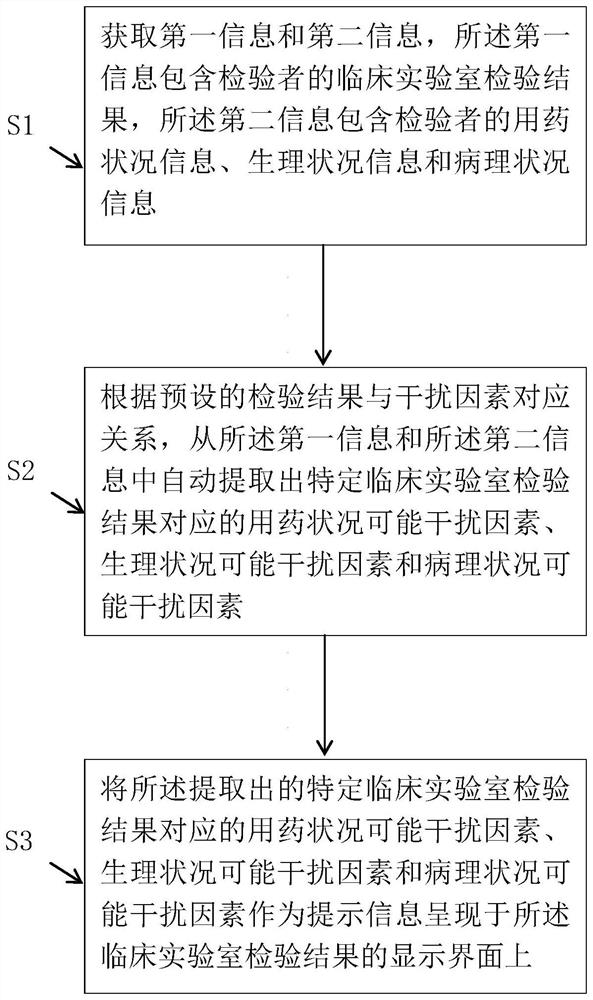
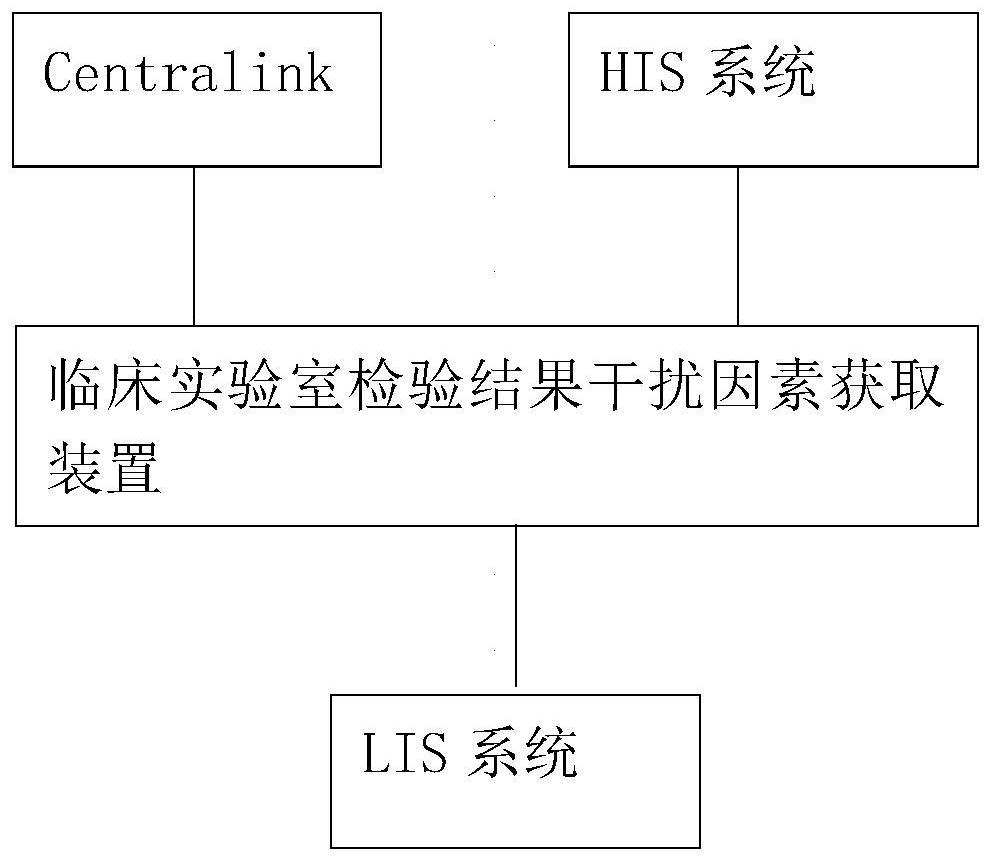
Clinical laboratory test result interference factor acquisition method and device and medium
PendingCN114550869AEasy to assistDrug and medicationsMedical practises/guidelinesMedicineEngineering
The invention discloses a clinical laboratory test result interference factor acquisition method and device and a computer readable storage medium, which can automatically extract a medication condition possible interference factor and / or a physiological condition possible interference factor and / or a pathological condition possible interference factor corresponding to a specific clinical laboratory test result. Comprising the steps that first information and second information are obtained, the first information comprises a clinical laboratory inspection result of an inspector, and the second information comprises at least one of medication condition information, physiological condition information and pathological condition information of the inspector; and according to a preset corresponding relationship between the test result and the interference factor, automatically extracting a drug use condition possible interference factor and / or a physiological condition possible interference factor and / or a pathological condition possible interference factor corresponding to the test result of the specific clinical laboratory from the first information and the second information.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
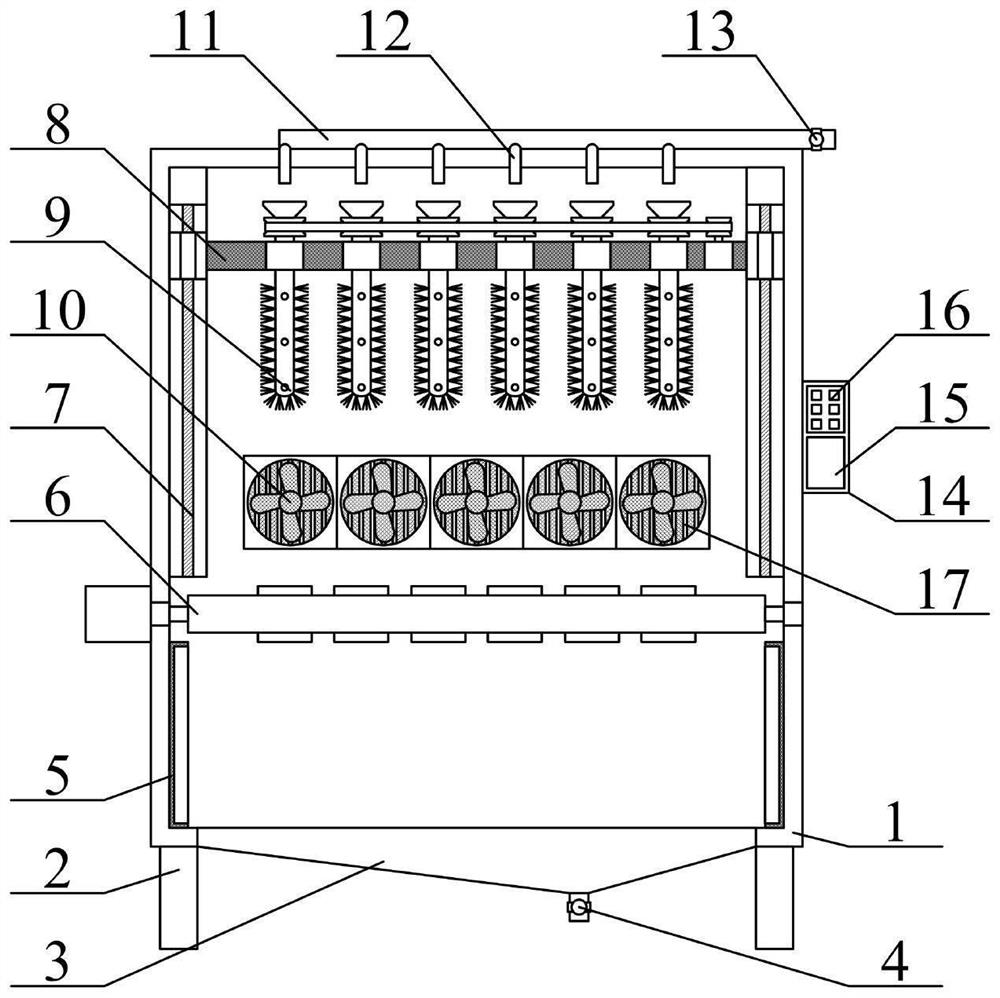
Intelligent test tube cleaning and drying all-in-one machine for clinical laboratory
The invention provides an intelligent clinical laboratory test tube cleaning and drying all-in-one machine which comprises a cleaning outer frame, supporting legs, a liquid storage hopper, a guide-out valve, a sterilizing lamp, a rotary storage frame structure, a lifting positioning frame structure, a positioning driving frame structure, a rotary cleaning frame structure, a fan, a cleaning liquid conveying pipe, a dropping pipette, a guide-in valve, a control frame, a controller, a control panel and an electric heating wire. The supporting legs are installed at the four corners of the lower portion of the cleaning outer frame through bolts. The liquid storage hopper is embedded into the middle lower part of the cleaning outer frame; the sterilizing lamps are embedded in the left side and the right side of the lower portion of the cleaning outer frame. An angle rotating motor is installed on the lower portion of the left side of the cleaning outer frame through screws, and test tubes in a transverse frame can be flexibly turned over in the using process. And the threaded groove is in threaded fit with the lifting screw rod, so that the heights of the positioning driving frame structure and the rotary cleaning frame structure can be flexibly adjusted according to requirements during use.
Owner:顾丽娜
Method for normalizing clinical laboratory measurements
ActiveUS8812241B1Microbiological testing/measurementDigital computer detailsCorrelation factorsPatient data
A computerized method for normalizing the results of clinical laboratory tests to a reference scale includes providing a measured value of a clinically significant parameter, providing a set of patient data, and providing a set of method data, including an indication of a method used by a testing instrument used to measure the measured value. One or more correlation factors are retrieved including a method correlation factor from a computer readable database based on the method data. The method correlation factor corresponds to the method used by the testing instrument used to measure the measured value. A normalized value of the clinically significant parameter is calculated based upon the one or more correlation factors. The normalized value may correspond to a value on the reference scale regardless of the method used by the testing instrument.
Owner:PRAIRIE VENTURES
Hospital clinical laboratory test tube batch taking equipment
The hospital clinical laboratory test tube batch taking equipment comprises a fixing frame and a fixing middle plate, the fixing middle plate is fixedly installed in the middle of the inner side of the fixing frame, and a first clamping plate used for clamping test tubes is movably installed on the side, close to the fixing middle plate, of the inner side of the fixing frame; a second clamping plate for clamping a test tube is movably installed on the other side, close to the fixed middle plate, of the inner side of the fixed frame, and a first supporting plate and a second supporting plate which are used in cooperation with the first clamping plate and the second clamping plate are movably installed on the inner side of the fixed middle plate; limiting clamping bases used for limiting the moving range of the first clamping plate and the second clamping plate are movably installed at the two ends of the fixing frame. By means of the arrangement of the first clamping plate and the second clamping plate, the batch taking device for the test tubes in the clinical laboratory of the hospital is provided with a double-row fixing structure, the fixing operation of the double-row test tubes can be completed at a time, and use is convenient.
Owner:王云鹏
Cell yield for synthetic tissue controls and synthetic tissue microarray controls
Pathology testing and clinical laboratory testing are important aspects of modern diagnostic and prognostic practices. Control samples are often used to maintain quality control (QC) for reproducibility of test results by immunohistochemical (IHC) staining, in situ hybridization (ISH), and other methods of molecular analyses. Some of the controls available for IHC staining and ISH staining of tumor tissues and other diseased tissues are cancer tissue-derived controls. However such types of controls are only available in very limited quantities, and once such controls are exhausted, replacementcontrols with the same characteristics may be unavailable. Other types of available controls are cancer cell lines-derived controls. However, such types of controls do not exhibit consistent patternsand levels of cellular expression of a given marker, or heterogeneity of said expression, which is ubiquitous to tumor tissues. As such, these controls have little or no morphological resemblance toactual tumor tissues. Further, conventional techniques to form controls involve processes that produce poor yield, thereby requiring significant amounts of cultured cells in order to form such controls, thereby increasing productivity costs and decreasing productivity efficiency.
Owner:SLMP LLC
A special disinfectant for laboratory testing instruments and preparation method thereof
ActiveCN104026173BExtended broad-spectrum sterilizationBroad-spectrum bactericidalBiocideFungicidesSmoked PlumDisinfectant
The invention discloses a disinfectant special for an inspection instrument in the inspection department and a preparation method of the disinfectant. The disinfectant is prepared mainly by the following steps of crushing pawpaw, fructus aurantii immaturus, schisandra chinensis, pericarpium citri reticulatae, smoked plum, chinese holly, pomegranate bark, exocarpium, eucalyptus robusta smith, dogwood, folium eriobotryae, selfheal, myrobalan, corn silk, ligusticum wallichii, glossy privet fruits and pericarpium citri reticulatae viride, soaking, heating and reflowing, wherein an auxiliary material is a filter liquor which is prepared by heating poinsettia, radix angelicae, hemp fimble stem-fibre, subprostrate sophora and fungus-infected rice spike with ethanol and extracting, wherein an additive of polyhexamethylene guanidine is added. The disinfectant is faintly acid, and the environmental acidity can be changed, so that most bacteria which grow best within a PH range of 6.0 to 8.0, have the strongest enzymatic activity and strongly grow and breed cannot exist, and the bacterial growth is effectively inhibited; the disinfectant is easily dissolved in water, and the disinfectant is easily washed out without residues after being used; the disinfectant has the advantages of being small in corrosivity, good in stability and simple in preparation process, can be widely applied to disinfecting the inspection instrument in the inspection department and is suitable for mass production.
Owner:湖州纯一生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com