Heparin content detection method
A detection method and heparin technology, applied in the field of biological detection, can solve the problems of accurate, reliable and simple detection results, and the inability to use automatic detection instruments.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The kit 1 provided by the present invention is prepared, comprising reagent 1 and reagent 2, and is used for determining the heparin content in blood.
[0092] Specifically: Reagent 1 activates the chromogenic substrate of factor X (FXa), and its specific preparation method is as follows: taking 4-Nz-D-Arg-Gly-Arg-p-nitroanilide 2HCl (S-2782) dissolved in a concentration of 20mM Tris buffer, then add hydrochloric acid to adjust the pH value to 7.4; then add sodium chloride and polyethylene glycol-8000, stir to obtain reagent 1, and its final concentrations are: 4-Nz-D-Arg-Gly -Arg-p-nitroanilide 2HCl 0.5 mM, NaCl 0.15 M, PEG-8000 1%.
[0093] Reagent 2 is activating factor X (FXa), and its specific preparation method is as follows: taking activating factor X (FXa) and dissolving it in a Tris buffer with a concentration of 20 mM, then adding hydrochloric acid to adjust the pH value to 7.4; then adding sodium chloride and polyethylene Diol-8000, and after stirring, reage...
Embodiment 2
[0103] The difference between this example and Example 1 is only that the heparin contained in the standard and sample described in Example 1 is Unfractionated heparin (UFH), while the heparin contained in the standard and sample described in this example is Low molecular weight heparin (low-molecular-weight heparin, LMWH). Other operations and proportions are the same as those in Example 1.
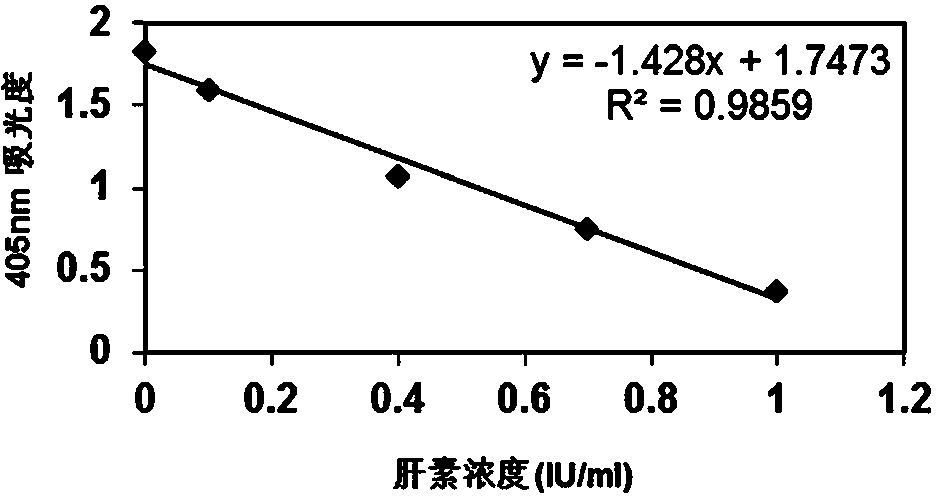
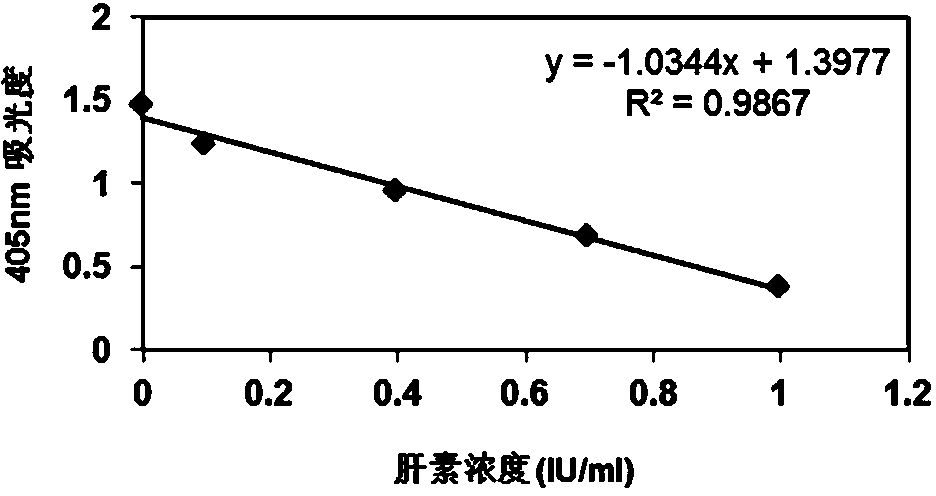
[0104] According to the gradient concentration of heparin standard solution and the corresponding absorbance value, a linear equation is used to draw a standard curve, please see the appendix figure 2 , its standard curve formula is y=-1.0344x+1.3977(R 2 =0.9867).
[0105] The specificity, sensitivity and linear range of the obtained kit are shown in the following experiments.
Embodiment 3
[0107] The kit 3 provided by the present invention is prepared, including Reagent 1, Reagent 2 and Reagent 3, and is used to measure the heparin content in blood. At this time, the measured heparin content needs to consider the endogenous antithrombin expression level of the sample.
[0108] Wherein, the preparation of reagent 1 and reagent 2 and the standard curve are the same as those in Example 1.
[0109] Reagent 3 is antithrombin, and its specific preparation method is as follows: dissolving antithrombin in a Tris buffer solution with a concentration of 20 mM, and then adding hydrochloric acid to adjust the pH value to 7.4. Then add sodium chloride and polyethylene glycol-8000 to obtain reagent 3, and its final concentrations are: antithrombin 0.2~0.5U / mL, sodium chloride 0.1~0.3M, polyethylene glycol 0.5~1% .
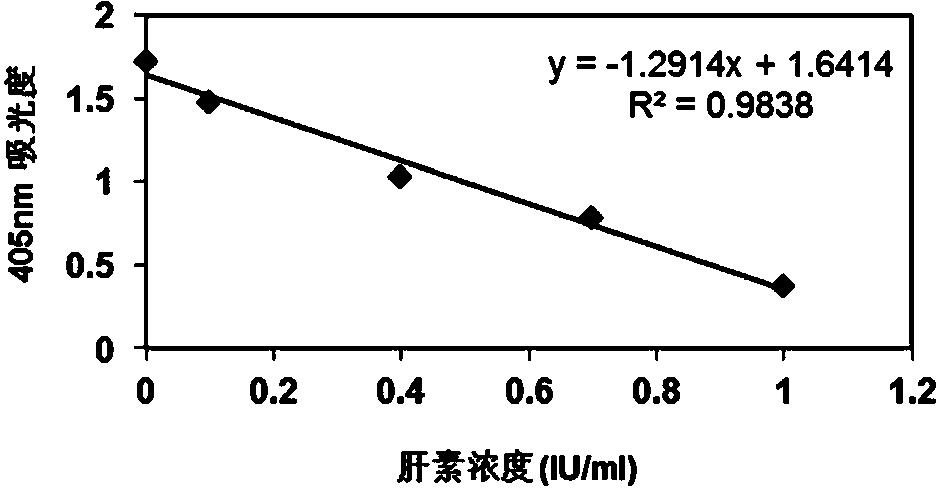
[0110] According to the gradient concentration of heparin standard solution and the corresponding absorbance value, a linear equation is used to draw a standard ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com