Cd47 antibodies, methods, and uses
A technology of antibody and antigen, applied in the field of CD47 antibody and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Example 1: Production of CD47 Antibody Using Hybridoma Technology
[0187] Immunization with recombinant human CD47-Fc chimera (R&D Systems) using Freund's adjuvant (Sigma), InterFAD (mouse interferon-α, PBL InterferonSource), or 2-dose adjuvant (Creative Diagnostics) using standard immunization protocols Balb / c mice were primed to develop anti-CD47 antibodies. To develop hybridomas expressing CD47 antibodies, spleens of immunized mice were harvested and B cells were isolated for fusion with SP20-Bcl2 myeloma cells. Hybridoma clones from the four fusions (C47Y1, C47Y2, C47Y3 and C47Y4) were analyzed by ELISA for antibodies binding to CD47 but not to the Fc-tag. Hybridoma supernatants showing specific binding to CD47 were further screened to bind to CD47-expressing Jurkat cells (TIB-152, ATCC) and to block the interaction of SIRP-α with Jurkat cells by meso scale discovery (MSD)-based assays. combined.
[0188] Briefly, Jurkat cells were washed and resuspended in ph...
Embodiment 2
[0192] Example 2: Production of CD47 Antibody Using Phage Display Technology
[0193] CD47 reagents and methods : Recombinant human CD47 extracellular domain (ECD) protein (SEQ ID NO. 22) was produced in-house with the addition of a C-terminal 6xHIS tag (SEQ ID NO: 55) for phage panning. A cDNA clone of human CD47 was purchased from Origene, and the ECD region was amplified by PCR and subcloned into a mammalian expression vector. After transient transfection of HEK 293F cells, secreted His-tagged human CD47-ECD protein was purified via immobilized metal affinity chromatography (IMAC) using a HisTrap column (GE Healthcare). Peak fractions were pooled and concentrated to obtain monomeric and dimeric forms of the CD47-ECD protein before a final polishing step by chromatography on a 26 / 60 Superdex 200 column (GE Healthcare). The CD47-ECD protein was biotinylated with a 10-fold molar excess of Thio-NHS-LC-Biotin (Pierce) for use in phage panning experiments.
[0194] Phage ...
Embodiment 3
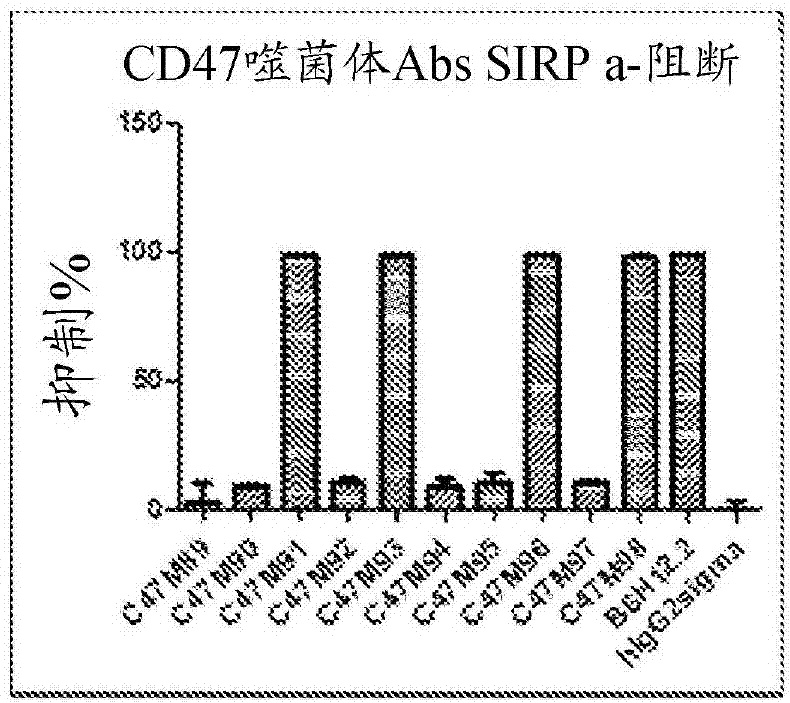
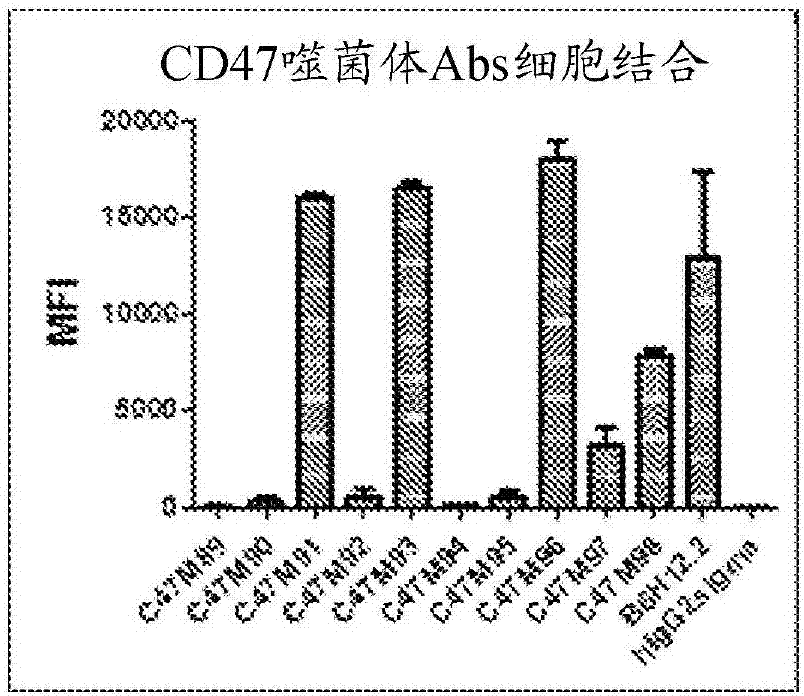
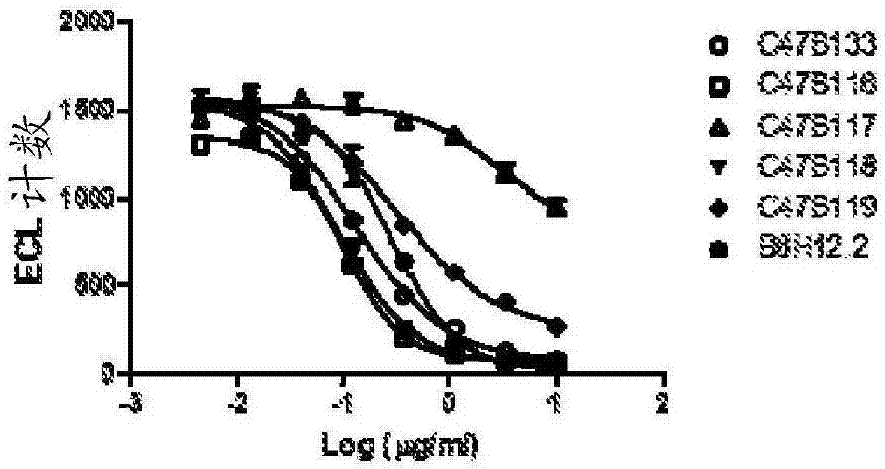
[0199] Example 3: Biological activity of CD47 antibodies
[0200] A total of 23 CD47 antibodies generated (20 from the hybridoma method and 3 from the phage display method) generated were analyzed for their ability to bind CD47 and block certain biological activities of CD47 using a variety of in vitro assays as described below.
[0201] CD47 binding assay : Human CD47 and Cynomolgus CD47 ECD proteins were produced in-house as His-tagged proteins as described in Example 2. The kinetic binding affinities of human CD47 and macaque CD47 ECD proteins were determined by Protein Interaction Array system (ProteOn). Briefly, mAbs were captured on sensor chips via anti-IgG-Fc to a surface density of 200-350 RU. The CD47 ECD monomeric protein was continuously titrated from 300 nM to 3.7 nM and injected for 5 min. Dissociation was monitored for 30 min. Data were fitted to a 1:1 binding model. K of 23 mAbs D Values and ratios between affinities to human and macaque CD47 protein...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com