Group I type 4 aviadenovirus strain WZ and application of strain WZ
A technology of poultry adenoviruses and strains, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of no related products, achieve good specificity, prevent poultry inclusion body hepatitis, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the above-mentioned virus solution comprising the avian adenovirus group I group 4 type 4 strain WZ strain according to claim 1, comprising the following steps: passing chicken embryos of the avian adenovirus group I group 4 type 4 virus strain WZ strain Inoculate SPF chickens with the virus, harvest the liver tissue of infected dead chickens, grind, freeze-thaw, and centrifuge, and the supernatant obtained is the virus liquid; or inoculate chicken embryo liver cells for culture, and harvest the cell supernatant after freezing and thawing. virus fluid.
[0039] Application of the above-mentioned avian adenovirus group I type 4 strain WZ strain in the preparation of group I group 4 avian adenovirus vaccine.
[0040] A poultry adenovirus group I type 4 virus vaccine, the active ingredient of the vaccine comprises the inactivated poultry adenovirus group I type 4 virus strain according to claim 1.
[0041]As in the above-mentioned preparation met...
Embodiment 1
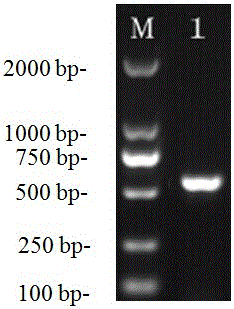
[0045] Application of the above-mentioned avian adenovirus group I type 4 strain WZ strain in the preparation of egg yolk antibody and antiserum for virus treatment. Example 1: Isolation and identification of group I group 4 avian adenovirus strain WZ strain
[0046] Since 2014, poultry inclusion body hepatitis and pericardial effusion syndrome infectious diseases have broken out in chicken flocks in many chicken raising areas in my country, with morbidity and mortality as high as 30%-70%, which has caused serious economic damage to the chicken industry. loss. The pathological changes at autopsy are mainly hepatitis, the liver is light in color, brittle, and enlarged, and there are hemorrhage points in the liver and skeletal muscle. Microscopic examination shows that there are large, round or irregular basophilic or eosinophilic nuclei in the liver cells. Body; pericardium all have varying degrees of effusion, and the effusion is light yellow or grass green, or jelly-like. Af...
Embodiment 2
[0077] Embodiment 2: Preparation of virus seed of group I group 4 avian adenovirus WZ strain
[0078] The FAdV 4 WZ strain virus strain that has been plaque-purified was expanded and cultured on chicken embryo liver cells by cell culture method, and the CCID of the WZ strain was determined at the same time 50 , the virus liquid obtained by cell culture at this time is the cell culture virus liquid. The cell culture virus liquid was prepared according to 10 6 .0 CCID 50 Intramuscularly inoculate 50-day-old SPF chickens with the same dose, harvest the liver tissue of the dead chickens, grind them, and centrifuge the supernatant to determine the virus content. The supernatant is the virus liquid used to prepare the vaccine. Will test for sterility and virus content ≥10 -7.5 CCID 50 The virus liquid was quantitatively subpackaged, freeze-dried and stored, and the original seed batch was established.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com