Chemiluminescent ligand analysis method for quantitative detection of human auto-antibody
An autoantibody and chemiluminescence technology, applied in the field of biomedicine, can solve the problems of inability to provide a reliable basis for autoimmune disease diagnosis and efficacy evaluation, and inability to accurately quantify autoantibodies, achieving high sensitivity, accuracy and specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
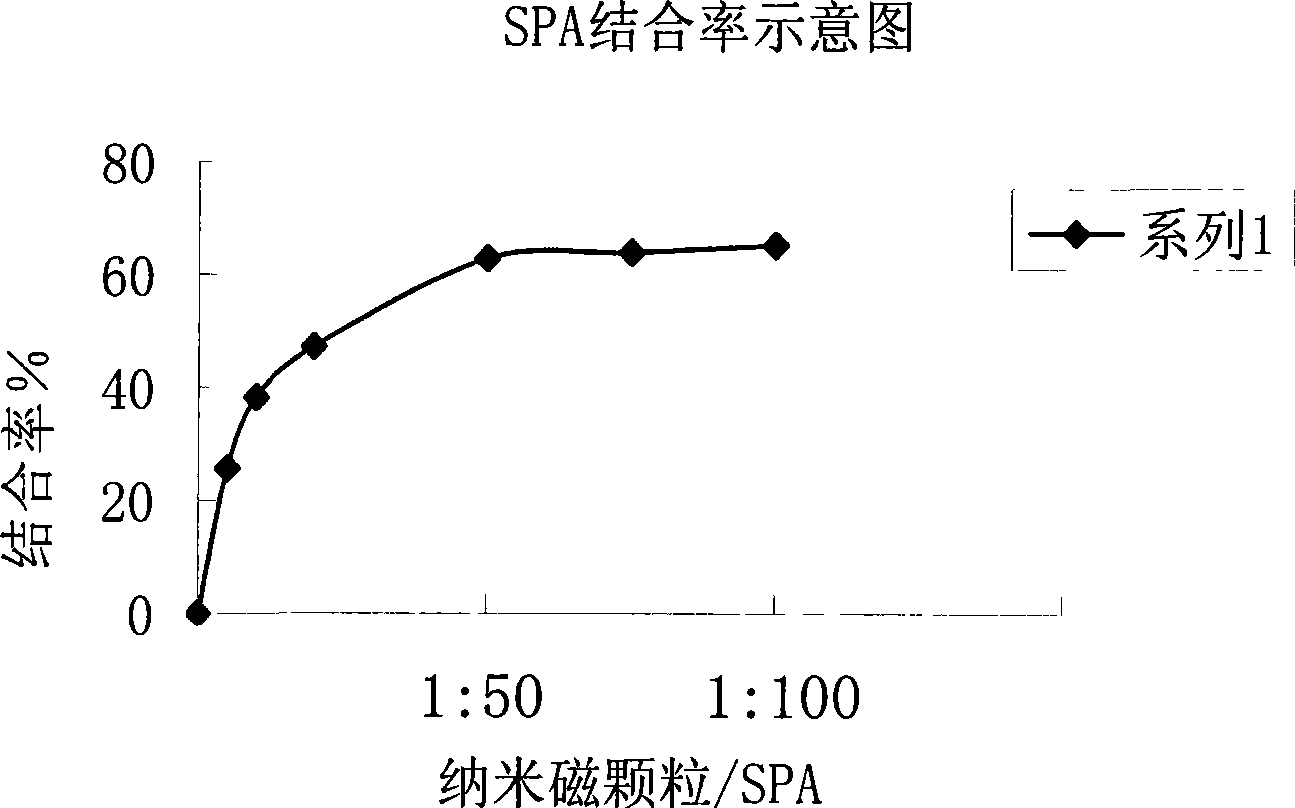
[0041] The content of this method is specified by the following examples:
[0042] Establishment of Quantitative Analysis Method for TPO Autoantibody Chemiluminescent Ligand (Nanomagnetic Particles)
[0043] (1) Preparation of each component of the kit:
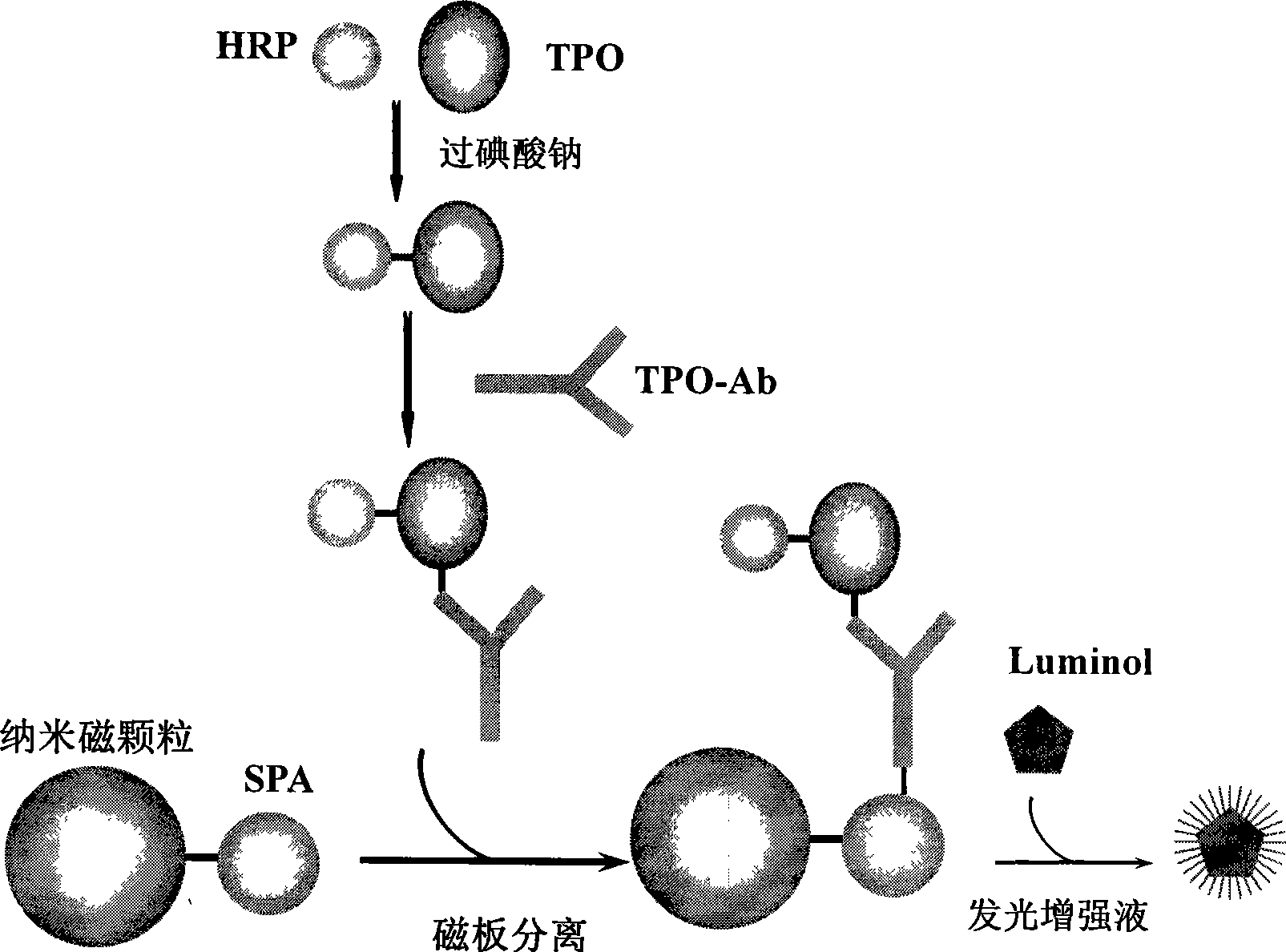
[0044] The main components of TPO-Ab chemiluminescence ligand quantitative analysis diagnostic kit are: marker (TPO-HRP), standard (TPO monoclonal antibody), ligand reagent (SPA-nano magnetic particles), enhanced chemiluminescence working solution ,detergent.
[0045] 1. Preparation and detection of labeled antigen (TPO-HRP):
[0046] a. Preparation: TPO-HRP was prepared by sodium periodate enzymatic labeling technique:
[0047] (1) Weigh 2.0mg of TPO into a 5ml glass bottle, add 1.0ml (0.05mol / L, pH7.5) carbonate buffer to dissolve, and store at 4°C for later use;
[0048] (2) Weigh 10.0mg of HRP into a 16×100mm glass test tube, add 1.0ml of deionized water, oscillate, after it is completely dissolved, take out 0.74ml an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com